Abstract
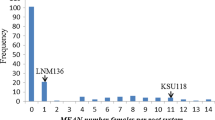
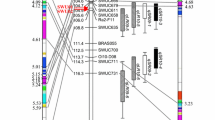
In rape (Brassica napus), no resistance to the beet cyst nematode (BCN) Heterodera schachtii is available. This study was carried out to determine the specific chromosome(s) of resistant radish (Raphanus sativus) carrying the gene(s) for nematode resistance as a prequisite to convert rape from a host into a trap crop for this pest. A Raphanobrassica progeny of 25 plants was analyzed which segregated for all nine chromosomes of the Raphanus genome in a genetic background of synthetic rape. The number of radish chromosomes was determined by fluorescence in situ hybridization, using the Raphanus-specific DNA probe pURsN; and their type was identified by chromosome-specific randomly amplified polymorphic DNA markers. Five different multiple rape–radish chromosome additions (comprising the whole set of nine radish chromosomes, a–i) were selected and crossed to rape. For each cross-progeny, the number of cysts on plant roots was counted 42 days after inoculation with a L2 larvae suspension. Simultaneously, the plants were characterized for the presence or absence of individual radish chromosomes, using sets of chromosome-specific markers. Thus, the effect of each radish chromosome on cyst number was tested. Chromosome d had a major resistance effect, whereas the presence/absence of the other radish chromosomes had nearly no influence on cyst number. Plants with added chromosome d showed a resistance level comparable with that of the radish donor parent. The analysis in the cross to rape of a plant monosomic only for chromosome d confirmed the strong effect of this chromosome on nematode resistance. A further experiment comprising seven crosses using winter rape breeding lines and monosomic addition line d as pollen parent provided the same results on a broader genetic basis. In each case, the added chromosome d in a single dosage caused nearly the full resistance of the radish donor. Resistance was independent of the glucosinolate content in the roots. The possibilities for stabilizing BCN resistance in rape and its use for other crops and nematodes are discussed.





Similar content being viewed by others
References
Baukloh H (1976) Untersuchungen zur Wirtspflanzeneignung der Kruziferen gegenüber dem Rübennematoden Heterodera schachtii (Schmidt), unter besonderer Berücksichtigung der Resistenzzüchtung. PhD thesis, Georg-August-Universität, Göttingen
Bünte R, Müller J, Friedt W (1997) Genetic variation and response to selection for resistance to root-knot nematodes in oil radish (Raphanus sativus ssp. oleiferus) Plant Breed 116:263–266
Chèvre AM, This P, Eber F, Deschamps M, Renard M, Delseny M, Quiros CF (1991) Characterization of disomic addition lines Brassica napus–Brassica nigra by isozyme, fatty-acid, and RFLP markers. Theor Appl Genet 81:43–49
Chèvre AM, Eber F, This P, Barret P, Tanguy X, Brun H, Delseny M, Renard M (1996) Characterization of Brassica nigra chromosomes and of blackleg resistance in B.napus–B.nigra addition lines. Plant Breed 115:113–118
Chèvre A.M, Eber F, Barret P, Dupuy P, Brace J. (1997) Identification of the different Brassica nigra chromosomes from both sets of B. oleracea–B. nigra and B. napus–B. nigra addition lines with a special emphasis on chromosome transmission and self-incompatibility. Theor Appl Genet 94:603–611
Clauß E (1978) Allohexaploide Gattungsbastarde vom Typ Brassico–Raphanobrassica. Arch Züchtungsforsch 5:297–302
Cook R, Rivoal R (1998) Genetics of resistance and parasitism. In: Sharma SB (ed) The cyst nematodes. Kluwer, Dordrecht, pp 322–352
Curi J, Zmoray I (1966): Beziehung klimatischer Faktoren zur Entwicklungsdauer des Rübennematoden Heterodera schachtii in der Slowakei (CSSR). Helmintologia 7:49–63
Dellaporta SJ, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Delourme R, Foisset N, Horvais R, Barret P, Champagne G, Cheung WY, Landry BS, Renard M (1998) Characterisation of the radish introgression carrying the Rfo restorer gene for the Ogu-INRA cytoplasmic male sterility in rapeseed (Brassica napus L.) Theor Appl Genet 97:129–134
Dolstra O (1982) Synthesis and fertility of X Brassicoraphanus and ways of transferring Raphanus characters to Brassica. (Agric Res Rep 917) Pudoc, Wageningen
Fatemy S, Abootorabi E (2002) Hatching activity, invasion rate and reproduction of Heterodera schachtii on oilseed rape cultivars. Nematol Mediterr 30:163–166
Gheysen G, Fenoll C (2002) Gene expression in nematode feeding sites. Annu Rev Phytopathol 40:191–219
Harrewijn JL (1987) Screening for resistance against the beet cyst nematode Heterodera schachtii Schm. in Brassica napus L. Meded Fac Landbouwwet Rijksuniver Gent 52:587–592
Hirai K, Irifune K, Tanaka R, Morikawa H (1995) Molecular and cytological characterization of a highly repeated DNA sequence in Raphanus sativus. Genome 38:1237–1243
Jung CR, Wehling, P, Löptien H (1986) Electrophoretic investigations on nematode resistant sugar beets. Plant Breed 97:39–45
Kaplan M, Caswell-Chen EP, Williamson VM (1999) Assessment of host-induced selection on three geographic isolates of Heterodera schachtii using RAPD and AFLP markers. Phytopathology 89:68–73
Lange W, Toxopheus H, Lubberts JH, Dolstra O, Harrewijen JL (1989) The development of raparadish (X Brassicaraphanus, 2n=38), a new crop in agriculture. Euphytica 40:1–4
Lazzeri L, Tacconi R, Palmieri S (1993) In vitro activity of some glucosinolates and their reaction products toward a population of the nematode Heterodera schachtii. J Agric Food Chem 41:825–829
Lelivelt CLC (1995) Attempted selection for partial resistance to the sugar-beet cyst nematode, Heterodera schachtii, in Brassica napus L. Fundam Appl Nematol 18:11–15
Lelivelt CLC, Hoogendoorn J (1993) The development of juveniles of Heterodera schachtii in roots of resistant and susceptible genotypes of Sinapis alba, Brassica napus, Raphanus sativus and hybrids. Neth J Plant Pathol 99:13–22
Lelivelt CLC, Krens FA (1992) Transfer of resistance to the beet cyst nematode (Heterodera schachtii Schm.) into the Brassica napus L. gene pool through intergeneric somatic hybridization with Raphanus sativus L. Theor Appl Genet 83:887–894
Lelivelt CLC, Leunissen EHM, Frederiks HJ, Helsper JPFG, Krens FA (1993a) Transfer of resistance to the beet cyst nematode (Heterodera schachtii SCHM) from Sinapis alba L (white mustard) to the Brassica napus L gene pool by means of sexual and somatic hybridization. Theor Appl Genet 85:688–696
Lelivelt CLC, Lange W, Dolstra O (1993b) Intergeneric crosses for the transfer of resistance to the beet cyst nematode from Raphanus sativus to Brassica napus. Euphytica 68:111–120
Löptien H (1984) Breeding nematode-resistant beets. I. Development of resistant alien additions by crosses between Beta vulgaris L. and wild species of the section Patellares. Z Pflanzenzuecht 92:208–220
Lubberts JH, Toxopeus H (1982) The development of beet cyst nematode-resistant fodder rape and white mustard (in Dutch). Zaadbelangen 36:66–69
Mesbah M, Scholten OE, Brock TSM de, Lange W (1997) Chromosomal localisation of genes for resistance to Heterodera schachtii, Cercospora beticola and Polymyxa betae using sets of Beta procumbens and B. patellaris derived monosomic additions in B. vulgaris. Euphytica 97:117–127
Metz PLJ, Nap JP, Stiekema WJ (1995) Hybridization of radish (Raphanus sativus L.) and oilseed rape (Brassica napus L.) through a flower-culture method. Euphytica 83:159–168
Mizushima U (1980) Genome analysis in Brassica and allied genera. In: Tsunoda S, Hinata K, Gómez-Campo C (eds) Brassica crops and wild allies—biology and breeding. Japan Scientific Societies Press, Tokyo, pp 89–106
Müller J (1985) Der Einfluss der Wirtspflanze auf die Geschlechtsdeterminierung bei Heterodera schachtii. Mitt Biol Bundesanst Land Forstwirtschaft, Berlin-Dahlem 226:46–63
Nielsen EL, Baltensperger DD, Kerr ED, Rife CL (2003) Host suitability of rapeseed for Heterodera schachtii. J Nematol 35:35–38
Riggs RD, Schuster RP (1998) Management. In: Sharma SB (ed) The cyst nematode. Kluwer, Dordrecht, pp 388–416
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Savitzky H (1975) Hybridization between Beta vulgaris and B. procumbens and transmission of nematode (Heterodera schachtii) resistance to sugar beet. Can J Genet Cytol 17:197–209
Schrader O, Budahn H, Ahne R (2000) Detection of 5S and 25S rRNA genes in Sinapis alba, Raphanus sativus and Brassica napus by double fluorescence in situ hybridization. Theor Appl Genet 100:665–669
Snowdon RJ, Winter H, Diestel A, Sacristán MD (2000) Development and characterisation of Brassica napus–Sinapis arvensis addition lines exhibiting resistance to Leptosphaeria maculans. Theor Appl Genet 101:1008–1014
Speckmann GJ, Bock TSM de (1982) The production of alien monosomic additions in Beta vulgaris as a source for introgressions of resistance to beet root nematode (Heterodera schachtii) from Beta species of the section Patellares. Euphytica 31:313–323
Struss D, Bellin U, Röbbelen G (1991) Development of B-genome chromosome addition lines of Brassica napus using different interspecific Brassica hybrids. Plant Breed 106:209–214
Sumbayak BS (1997) Untersuchungen zur Bedeutung der Wurzelglucosinolate im Hinblick auf Nematodenresistenz bei Ölrettich (Raphanus sativus) und Raps (Brassica napus L.) und deren Kreuzungsnachkommenschaften. PhD thesis, Justus-Liebig-Universität, Giessen
Thierfelder A (1994) Genetische Untersuchungen für die züchterische Entwicklung neuer Rapssorten (Brassica napus L.) mit Resistenz gegen Nematoden (Heterodera schachtii Schm.). Shaker, Aachen
Thies W (1979) Quantitative analysis of glucosinolates after their enzymatic desulfatation on ion exchange columns. Proc Int Rapeseed Conf 5:136–39
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Williamson VM, Hussey RS (1996) Nematode pathogenesis and resistance in plants. Plant Cell 8:1735–1745
Zhu JS, Struss D, Röbbelen G (1993) Studies on resistance to Phomalingam in Brassica napus–Brassica nigra addition lines. Plant Breed 111:192–197
Acknowledgements
We thank Prof. Clauß for supplying the Raphanobrassica material and M. Schlathölter (P.H. Petersen Saatzucht Lundsgaard) for supplying infection material. We are grateful to H. Heinrich, A. Garve and K. Meyer for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Möllers
Rights and permissions
About this article
Cite this article
Peterka, H., Budahn, H., Schrader, O. et al. Transfer of resistance against the beet cyst nematode from radish (Raphanus sativus) to rape (Brassica napus) by monosomic chromosome addition. Theor Appl Genet 109, 30–41 (2004). https://doi.org/10.1007/s00122-004-1611-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-004-1611-2