Abstract
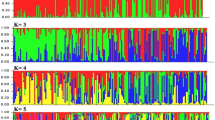
The majority of 170 rice accessions used in this study were diverse landraces or varieties from a putative mini-core collection of Chinese germplasm along with some widely used parental lines in genetic analysis or breeding (a few from abroad). The population was genotyped using 84 SSR or InDel markers on chromosome 7 and 48 markers on other chromosomes. The phenotyping of heading date, plant height and panicle length were carried out in different locations for 2 years. Based on morphological characterization, distance-based clustering and model-based estimation of marker data, the population showed a predominant structure with two subpopulations in correspondence with indica and japonica subspecies. The estimation of linkage disequilibrium in 2 Mb windows varied along chromosome 7 and showed parallel changes with inter-subspecies differentiation of marker loci (Fst). Based on the mixed linear model considering population structure and family relatedness [i.e. the (Q + K) model], one to three associated markers (P ≤ 0.0001) per trait per experiment were scanned out on rice chromosome 7. Most significant loci were repeated for the data from both field experiments while two loci were associated with two or three traits. Marker-based allelic effects were shown in a couple of associated markers as examples. The application of association results in breeding program was also discussed.




Similar content being viewed by others
References
Agrama HA, Eizenga GC, Yan W (2007) Association mapping of yield and its components in rice cultivars. Mol Breed 19:341–356
Aranzana MJ, Kim S, Zhao K, Bakker E, Horton M, Jakob K, Lister C, Molitor J, Shindo C, Tang C, Toomajian C, Traw B, Zheng H, Bergelson J, Dean C, Marjoram P, Nordborg M (2005) Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes. PLoS Genet 1:531–539
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635
Breseghello F, Sorrells ME (2006) Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172:165–1177
Caldwell KS, Russell J, Langridge P, Powell W (2006) Extreme population-dependent linkage disequilibrium detected in an inbreeding plant species, Hordeum vulgare. Genetics 172:557–567
Chen X, Temnykh S, Xu Y, Cho XG, McCouch SR (1997) Development of a microsatellite framework map providing genome-wide coverage in rice (Oryza sativa L.). Theor Appl Genet 95:553–567
Cheng KS (1985) A statistical evaluation of the classification of rice cultivars into hsien and keng subspecies. Rice Newsl 2:46–48
Colosi JC, Schaal BA (1993) Tissue grinding with ball bearings and vortex mixer for DNA extraction. Nucleic Acids Res 21:1051–1052
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Flint-Garcia SA, Thornsberry JM, Buckler ES (2003) Structure of linkage disequilibrium in plants. Ann Rev Plant Biol 54:357–374
Flint-Garcia SA, Thuillet AC, Yu J, Pressoir G, Romero SM, Mitchell SE, Doebley J, Kresovich S, Goodman MM, Buckler ES (2005) Maize association population: a high-resolution platform for quantitative trait locus dissection. Plant J 44:1054–1064
Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S (2005) Genetic structure and diversity in Oryza sativa L. Genetics 169:1631–1638
Hao CY, Dong YC, Wang LF, Zhang HN, Ge HM, Jia JZ, Zhang XY (2008) Genetic diversity and construction of core collection in Chinese wheat genetic resources. Chin Sci Bull 53:1518–1526
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hasan M, Friedt W, Pons-Kühnemann J, Freitag NM, Link K, Snowdon RJ (2008) Association of gene-linked SSR markers to seed glucosinolate content in oilseed rape (Brassica napus ssp. napus). Theor Appl Genet 116:1035–1049
Holland JB (2007) Genetic architecture of complex traits in plants. Curr Opin Plant Biol 10:156–161
Hyten DL, Choi IY, Song Q, Shoemaker RC, Nelson RL, Costa JM, Specht JE, Cregan PB (2007) Highly variable patterns of linkage disequilibrium in multiple soybean populations. Genetics 175:1937–1944
Insightful Corporation (2001) S-Plus 6 for windows, guide to statistics, vol 1. Insightful Corporation, Seattle
Iwata H, Uga Y, Yoshioka Y, Ebana K, Hayashi T (2007) Bayesian association mapping of multiple quantitative trait loci and its application to the analysis of genetic variation among Oryza sativa L. germplasms. Theor Appl Genet 114:1437–1449
Jiang GH, Xu CG, Li XH, He YQ (2004) Characterization of the genetic basis for yield and its component traits of rice revealed by doubled haploid population. Acta Genet Sin 31:63–72
Kato S, Kosaka H, Hara S (1928) On the affinity of rice varieties as shown by fertility of hybrid plants. Bull Sci Fac Agric Kyushu Univ 3:132
Kraakman AT, Niks RE, Van den Berg PM, Stam P, Van Eeuwijk FA (2004) Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics 168:435–446
Li ZC, Zhang HL, Zeng YW, Yang ZY, Shen SQ, Sun CQ, Wang XK (2002) Studies on sampling schemes for the establishment of core collection of rice landraces in Yunnan, China. Genet Resour Crop Evol 49:67–74
Li ZK, Yu SB, Lafitte HR, Huang N, Courthouse B, Hittalmani S, Vijayakumar CH, Liu GF, Wang GC, Shashidhar HE, Zhuang JY, Zheng KL, Singh VP, Sidhu JS, Srivantaneeyakul S, Khush GS (2003) QTL environment interactions in rice. I. Heading date and plant height. Theor Appl Genet 108:141–153
Li YH, Guan RX, Liu ZX, Ma YS, Wang LX, Li LH, Lin FY, Luan WJ, Chen PY, Yan Z, Guan Y, Zhu L, Ning XC, Smulders MJ, Li W, Piao RH, Cui YH, Yu ZM, Guan M, Chang RZ, Hou AF, Shi AN, Zhang B, Zhu SL, Qiu LJ (2008) Genetic structure and diversity of cultivated soybean (Glycine max (L.) Merr.) landraces in China. Theor Appl Genet 117:857–871
Lin SY, Sasaki T, Yano M (1998) Mapping quantitative trait loci controlling seed dormancy and heading date in rice. Oryza sativa L., using backcross inbred lines. Theor Appl Genet 96:997–1003
Liu K, Muse M (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Loiselle BA, Sork VL, Nason J, Graham C (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425
Lu CF, Shen LH, Tan ZB, Xu YB, He P, Chen Y, Zhu LH (1997) Comparative mapping of QTLs for agronomic traits of rice across environments by using a doubled-haploid population. Theor Appl Genet 94:145–154
Mather KA, Caicedo AL, Polato NR, Olsen KM, McCouch S, Purugganan MD (2007) The extent of linkage disequilibrium in rice (Oryza sativa L.). Genetics 177:2223–2232
Mazzucato A, Papa R, Bitocchi E, Mosconi P, Nanni L, Negri V, Picarella ME, Siligato F, Soressi GP, Tiranti B, Veronesi F (2008) Genetic diversity, structure and marker-trait associations in a collection of Italian tomato (Solanum lycopersicum L.) landraces. Theor Appl Genet 116:657–669
Mei HW, Feng FJ, Lu BR, Wen WW, Paterson AH, Cai XX, Chen L, Feltus FA, Xu XY, Wu JH, Yu XQ, Chen HW, Li Y, Luo LJ (2007) Experimental validation of inter-subspecific genetic diversity in rice represented by the differences between the DNA sequences of ‘Nipponbare’ and ‘93-11’. Chin Sci Bull 52:1327–1337
Pritchard JK, Stephen M, Donnelly P (2000a) Inference on population structure using multilocus genotype data. Genetics 155:945–959
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000b) Association mapping in structured populations. Am J Hum Genet 67:170–181
Remington DL, Thornsberry JM, Matsuoka Y, Wilson LM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler ES (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci 20:11479–11484
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco
Somers DJ, Banks T, DePauw R, Fox S, Clarke J, Pozniak C, McCartney C (2007) Genome-wide linkage disequilibrium analysis in bread wheat and durum wheat. Genome 50:557–567
Thornsberry JM, Goodman MM, Doebley J, Kresovich S, Nielsen D, Buckler ES (2001) Dwarf8 polymorphisms associate with variation in flowering time. Nat Genet 28:86–289
Wang XK, Li RH, Sun CQ, Li ZC, Cai HW, Sun XL (1998) Identification and classification of subspecies of Asian cultivated rice and their hybrids. Chin Sci Bull 43:1864–1872
Wang RH, Yu YT, Zhao JR, Shi YS, Song YC, Wang TY, Li Y (2008) Population structure and linkage disequilibrium of a mini core set of maize inbred lines in China. Theor Appl Genet 117:1141–1153
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Xu JL, Xue QZ, Luo LJ, Li ZK (2001) QTL dissection of panicle number per plant and spikelet number per panicle in rice (Oryza sativa L.). Acta Genet Sinica 28:752–759
Xu Y, Beachell H, McCouch SR (2004) A marker-based approach to broadening the genetic base of rice in the USA. Crop Sci 44:1947–1959
Xue WY, Xing YZ, Weng XY, Zhao Y, Tang WJ, Wang L, Zhou HJ, Yu SB, Xu CG, Li XH, Zhang QF (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yan JQ, Zhu J, He C, Benmoussa M, Wu P (1998) Molecular dissection of developmental behavior of plant height in rice (Oryza sativa L.). Genetics 150:1257–1265
Yu JM, Pressoir G, Briggs WH, Vroh Bi I, Yamasaki M, Doebley JF, McMullen MD, Gaut BS, Nielsen DM, Holland JB, Kresovich S, Buckler ES (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38:203–208
Zhang N, Xu Y, Akash M, McCouch S, Oard JH (2005) Identification of candidate markers associated with agronomic traits in rice using discriminant analysis. Theor Appl Genet 110:721–729
Zhu CS, Gore M, Buckler ES, Yu JM (2008) Status and prospects of association mapping in plants. Plant Genome 1:5–20
Acknowledgments
The authors are grateful to Dr. Zichao Li for providing us the rice accessions in the putative mini-core collection of Chinese rice germplasm. Two anonymous reviewers gave us many critical comments, based on that the manuscript was improved in several issues from its early version. This study was jointly supported by a grant from the Ministry of Agriculture of China (948-2006-R1), a grant from National Key Basic Research Program of China (973 Plan), a grant from the National Natural Science Foundation of China (30830071) and a grant from Shanghai Municipal Commission of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by J. Yu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, W., Mei, H., Feng, F. et al. Population structure and association mapping on chromosome 7 using a diverse panel of Chinese germplasm of rice (Oryza sativa L.). Theor Appl Genet 119, 459–470 (2009). https://doi.org/10.1007/s00122-009-1052-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-1052-z