Abstract
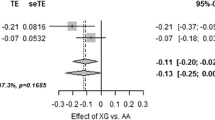
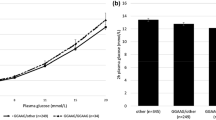
The liver X receptors (LXRs)-α and -β play a crucial role in control of insulin production and secretion in pancreatic β-cells. We hypothesized that common variants in the NR1H2 and NR1H3 genes, encoding LXR-β and -α, respectively, may alter pancreatic β-cell function. One thousand five hundred seventy-four subjects of European ancestry with elevated risk for type 2 diabetes were genotyped for the two NR1H2 single nucleotide polymorphisms (SNPs) rs2248949 and rs1405655 and for the four NR1H3 SNPs rs11039149, rs3758673, rs12221497 and rs2279238, and association studies with metabolic traits were performed. Metabolic characterization comprised an oral glucose tolerance test (OGTT) in all participants and, in addition, a hyperinsulinemic–euglycemic clamp and an intravenous glucose tolerance test (IVGTT) in subsets. One hundred per cent of common genetic variation (minor allele frequency ≥1%) within the NR1H2 and NR1H3 loci (D′ = 1.0; r² ≥ 0.8) were covered by the six chosen tagging SNPs. NR1H2 rs2248949 was nominally associated with OGTT-derived first-phase insulin secretion and proinsulin conversion to insulin and significantly associated with the AUC of insulin levels during the IVGTT (p = 0.007) after adjustment for age, gender, BMI and insulin sensitivity in the dominant model, with the minor allele conferring reduced pancreatic β-cell function to the carriers. In subjects of European ancestry at increased risk for type 2 diabetes, common variation within the NR1H2 gene impaired insulin secretion, which may facilitate the development of type 2 diabetes.

Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- GWA:
-
Genome-Wide Association
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- ISI:
-
Insulin sensitivity index
- LD:
-
Linkage disequilibrium
- LXR:
-
Liver X receptor
- MADD:
-
Mitogen-activated protein kinase-activating death domain
- MAF:
-
Minor allele frequency
- MAPK:
-
Mitogen-activated protein kinase
- NR1H2:
-
Nuclear receptor subfamily 1, group H, member 2
- NR1H3:
-
Nuclear receptor subfamily 1, group H, member 3
- OGTT:
-
Oral glucose tolerance test
- SNP:
-
Single nucleotide polymorphism
References
McCarthy MI, Hattersley AT (2008) Learning from molecular genetics: novel insights arising from the definition of genes for monogenic and type 2 diabetes. Diabetes 57:2889–2898
Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341
Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU et al (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345
Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G et al (2008) Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645
van Hoek M, Dehghan A, Witteman JC, van Duijn CM, Uitterlinden AG, Oostra BA, Hofman A, Sijbrands EJ, Janssens AC (2008) Predicting type 2 diabetes based on polymorphisms from genome-wide association studies: a population-based study. Diabetes 57:3122–3128
Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DP, Holmkvist J, Borch-Johnsen K, Jorgensen T et al (2008) SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 40:1098–1102
Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y et al (2008) Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40:1092–1097
Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU et al (2010) Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 42:142–148
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL et al (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42:105–116
Ingelsson E, Langenberg C, Hivert MF, Prokopenko I, Lyssenko V, Dupuis J, Magi R, Sharp S, Jackson AU, Assimes TL et al (2010) Detailed physiologic characterization reveals diverse mechanisms for novel genetic loci regulating glucose and insulin metabolism in humans. Diabetes 59:1266–1275
Qi L, Cornelis MC, Kraft P, Stanya KJ, Kao WH, Pankow JS, Dupuis J, Florez JC, Fox CS, Pare G et al (2010) Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet 19:2706–2715
Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G et al (2010) Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42:579–589
Florez JC (2008) Clinical review: the genetics of type 2 diabetes: a realistic appraisal in 2008. J Clin Endocrinol Metab 93:4633–4642
Kalaany NY, Mangelsdorf DJ (2006) LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68:159–191
Efanov AM, Sewing S, Bokvist K, Gromada J (2004) Liver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cells. Diabetes 53(Suppl 3):S75–S78
Steffensen KR, Gustafsson JA (2004) Putative metabolic effects of the liver X receptor (LXR). Diabetes 53(Suppl 1):S36–S42
Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, Zhang Y, Stayrook KR, Suen C, Otto KA et al (2003) Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J Biol Chem 278:1131–1136
Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL et al (2003) Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424
Grefhorst A, van Dijk TH, Hammer A, van der Sluijs FH, Havinga R, Havekes LM, Romijn JA, Groot PH, Reijngoud DJ, Kuipers F (2005) Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am J Physiol Endocrinol Metab 289:E829–E838
Gerin I, Dolinsky VW, Shackman JG, Kennedy RT, Chiang SH, Burant CF, Steffensen KR, Gustafsson JA, MacDougald OA (2005) LXRbeta is required for adipocyte growth, glucose homeostasis, and beta cell function. J Biol Chem 280:23024–23031
Meng ZX, Nie J, Ling JJ, Sun JX, Zhu YX, Gao L, Lv JH, Zhu DY, Sun YJ, Han X (2009) Activation of liver X receptors inhibits pancreatic islet beta cell proliferation through cell cycle arrest. Diabetologia 52:125–135
Choe SS, Choi AH, Lee JW, Kim KH, Chung JJ, Park J, Lee KM, Park KG, Lee IK, Kim JB (2007) Chronic activation of liver X receptor induces beta-cell apoptosis through hyperactivation of lipogenesis: liver X receptor-mediated lipotoxicity in pancreatic beta-cells. Diabetes 56:1534–1543
The International HapMap Project (2003) Nature 426:789–796
Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, Spieth C, Weigert C, Fritsche A, Stumvoll M et al (2005) Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia 48:2282–2291
Fritsche A, Madaus A, Stefan N, Tschritter O, Maerker E, Teigeler A, Haring H, Stumvoll M (2002) Relationships among age, proinsulin conversion, and beta-cell function in nondiabetic humans. Diabetes 51(Suppl 1):S234–S239
Schievella AR, Chen JH, Graham JR, Lin LL (1997) MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem 272:12069–12075
Acknowledgements
We thank all study participants for their cooperation. We thank the International HapMap Consortium for the public allocation of genotype data. We gratefully acknowledge the excellent technical assistance of Anna Bury, Alke Guirguis, Heike Luz, Melanie Weisser and Roman Werner. The study was supported by a grant from the German Research Foundation (FR 1561/5-1) and the German Federal Ministry for Education and Research (DLR 01GI0925).
Disclosure statement
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Caroline Ketterer and Karsten Müssig contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 69 kb)
Rights and permissions
About this article
Cite this article
Ketterer, C., Müssig, K., Machicao, F. et al. Genetic variation within the NR1H2 gene encoding liver X receptor β associates with insulin secretion in subjects at increased risk for type 2 diabetes. J Mol Med 89, 75–81 (2011). https://doi.org/10.1007/s00109-010-0687-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0687-1