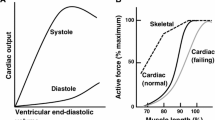
Abstract
The cardiac myofilaments are composed of highly ordered arrays of proteins that coordinate cardiac contraction and relaxation in response to the rhythmic waves of [Ca2+] during the cardiac cycle. Several cardiac disease states are associated with altered myofilament protein interactions that contribute to cardiac dysfunction. During acute myocardial ischemia, the sensitivity of the myofilaments to activating Ca2+ is drastically reduced, largely due to the effects of intracellular acidosis on the contractile machinery. Myofilament Ca2+ sensitivity remains compromised in post-ischemic or “stunned” myocardium even after complete restoration of blood flow and intracellular pH, likely because of covalent modifications of or proteolytic injury to contractile proteins. In contrast, myofilament Ca2+ sensitivity can be increased in chronic heart failure, owing in part to decreased phosphorylation of troponin I, the inhibitory subunit of the troponin regulatory complex. We highlight, in this paper, the central role of the myofilaments in the pathophysiology of each of these distinct disease entities, with a particular focus on the molecular switch protein troponin I. We also discuss the beneficial effects of a genetically engineered cardiac troponin I, with a histidine button substitution at C-terminal residue 164, for a variety of pathophysiologic conditions, including hypoxia, ischemia, ischemia–reperfusion and chronic heart failure.




Similar content being viewed by others
References
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415(6868):198–205
Orchard CH, Kentish JC (1990) Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol 258:C967–C981
Bolli R, Marban E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79(2):609–634
Piacentino V III et al (2003) Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res 92(6):651–658
Morgan JP (1991) Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med 325(9):625–632
Perez NG et al (1999) Origin of contractile dysfunction in heart failure: calcium cycling versus myofilaments. Circulation 99(8):1077–1083
Brixius K et al (2002) Increased Ca2+-sensitivity of myofibrillar tension in heart failure and its functional implication. Basic Res Cardiol 97(Suppl 1):I111–I117
Wolff MR et al (1996) Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest 98(1):167–176
Noguchi T et al (2004) Thin filament-based modulation of contractile performance in human heart failure. Circulation 110:982–987
VanBuren P, Okada Y (2005) Thin filament remodeling in failing myocardium. Heart Fail Rev 10(3):199–209
LeWinter MM (2005) Functional consequences of sarcomeric protein abnormalities in failing myocardium. Heart Fail Rev 10(3):249–257
Metzger JM, Westfall MV (2004) Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res 94:146–158
Thom T et al (2006) Heart disease and stroke statistics—2006 update. A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation 113:e85
Farah CS, Reinach FC (1995) The troponin complex and regulation of muscle contraction. FASEB J 9(9):755–767
Tobacman LS (1996) Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol 58:447–481
Takeda S et al (2003) Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 424:35–41
Li MX, Spyracopoulos L, Sykes BD (1999) Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry 38(26):8289–8298
Li MX, Wang X, Sykes BD (2004) Structural based insights into the role of troponin in cardiac muscle pathophysiology. J Muscle Res Cell Motil 25(7):559–579
Pirani A et al (2006) An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol 357(3):707–717
Vinogradova MV et al (2005) Ca(2+)-regulated structural changes in troponin. Proc Natl Acad Sci USA 102(14):5038–5043
Hoffman RM, Blumenschein TM, Sykes BD (2006) An interplay between protein disorder and structure confers the Ca2+ regulation of striated muscle. J Mol Biol 361(4):625–633
Murakami K et al (2005) Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol 352(1):178–201
Saggin L et al (1989) Troponin I switching in the developing heart. J Biol Chem 264(27):16299–16302
Reiser PJ et al (1994) Tension production and thin-filament protein isoforms in developing rat myocardium. Am J Physiol 36:H1589–H1596
Siedner S et al (2003) Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J Physiol 548(Pt 2):493–505
Hunkeler NM, Kullman J, Murphy AM (1991) Troponin I isoform expression in human heart. Circ Res 69(5):1409–1414
Kruger M, Kohl T, Linke WA (2006) Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol Heart Circ Physiol 291(2):H496–H506
Westfall MV et al (2001) Troponin I chimera analysis of the cardiac myofilament tension response to protein kinase A. Am J Physiol 280:C324–C332
Westfall MV, Metzger JM (2001) Troponin I isoforms and chimeras: tuning the molecular switch of cardiac contraction. News Phyiol Sci 16:278–281
Westfall MV, Rust EM, Metzger JM (1997) Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc Natl Acad Sci 94:5444–5449
Wolska BM et al (2001) Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol 536(3):863–870
Westfall MV, Albayya FP, Metzger JM (1999) Functional analysis of troponin I regulatory domains in the intact myofilament of adult single cardiac myocytes. J Biol Chem 274(32):22508–22516
Westfall MV et al (2000) Chimera analysis of troponin I domains that influence Ca2+-activated myofilament tension in adult cardiac myocytes. Circ Res 86:470–477
Westfall MV, Rust EM, Metzger JM (2001) Specific charge differences in troponin I isoforms influence myofilament calcium sensitivity of tension in adult cardiac myocytes. Biophys J 80:356A
Dargis R et al (2002) Single mutation (A162H) in human cardiac troponin I corrects acid pH sensitivity of Ca2+-regulated actomyosin S1 ATPase. J Biol Chem 277(38):34662–34665
Day SM et al (2006) Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med 12(2):181–189
Katz AM et al (2001) Physiology of the heart. Lippincott Williams and Wilkins, Philadelphia, pp 630–657
Solaro RJ et al (1988) Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ Res 63:779–787
Kim SJ, Depre C, Vatner SF (2003) Novel mechanisms mediating stunned myocardium. Heart Fail Rev 8(2):143–153
Gao WD et al (1996) Intrinsic myofilament alterations underlying the decreased contractility of stunned myocardium. Circ Res 78:455–465
Van Eyk JE et al (1998) Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts. Circ Res 82:261–271
Zhao K et al (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279(33):34682–34690
McDonough JL, Arrell DK, Van Eyk JE (1999) Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res 84:9–20
Murphy AM et al (2000) Transgenic mouse model of stunned myocardium. Science 287:488–491
Thomas SA et al (1999) Absence of troponin I degradation or altered sarcoplasmic reticulum uptake protein expression after reversible ischemia in swine. Circ Res 85:446–456
Canty JM, Lee TC (2002) Troponin I proteolysis and myocardial stunning: now you see it—now you don’t. J Mol Cell Cardiol 34:375–377
Feng J et al (2001) Preload induces troponin I degradation independently of myocardial ischemia. Circulation 103:2035–2037
Gonzalez MR, Pharmacologic AD (2006) Treatment of heart failure due to ventricular dysfunction by myocardial stunning. Am J Cardiovasc Drugs 6(2):69–75
Piper HM, Meuter K, Schafer C (2003) Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg 75:S644–S648
Garcia-Dorado D (2004) Myocardial reperfusion injury: a new view. Cardiovasc Res 61(3):363–364
Ohtsuka M et al (2004) Role of Na+–Ca2+ exchanger in myocardial ischemia/reperfusion injury: evaluation using a heterozygous Na+–Ca2+ exchanger knockout mouse model. Biochem Biophys Res Commun 314(3):849–853
Stromer H et al (2000) Na(+)/H(+) exchange inhibition with HOE642 improves postischemic recovery due to attenuation of Ca(2+) overload and prolonged acidosis on reperfusion. Circulation 101(23):2749–2755
Taylor MD et al (2005) Ethyl pyruvate enhances ATP levels, reduces oxidative stress and preserves cardiac function in a rat model of off-pump coronary bypass. Heart Lung Circ 14(1):25–31
Cross HR et al (2002) Ablation of PLB exacerbates ischemic injury to a lesser extent in female and male mice: protective role of NO. Am J Physiol 284:H683–H690
del Monte F et al (2004) Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci 101:5622–5627
Pieske B, Maier LS, Schmidt-Schweda S (2002) Sarcoplasmic reticulum Ca2+ load in human heart failure. Basic Res Cardiol 97(Suppl 1):I63–I71
Pieske B et al (1999) Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85:38–46
Maier LS et al (2003) Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92(8):904–911
Baartscheer A et al (2003) SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res 58(1):99–108
Hobai IA, O’Rourke B (2001) Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation–contraction coupling in canine heart failure. Circulation 103(11):1577–1584
Hasenfuss G (1998) Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 37(2):279–289
Hasenfuss G et al (1997) Calcium handling proteins in the failing human heart. Basic Res Cardiol 92(Suppl 1):87–93
MacLennan DH, Kranias EG (2003) Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4(7):566–577
Wehrens XH, Marks AR (2004) Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat Rev Drug Discov 3(7):565–573
Hoshijima M (2005) Gene therapy targeted at calcium handling as an approach to the treatment of heart failure. Pharmacol Ther 105(3):211–228
Haghighi K et al (2006) A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA 103(5):1388–1393
Haghighi K et al (2003) Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111(6):869–876
Nagueh SF et al (2004) Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 110(2):155–162
Makarenko I et al (2004) Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res 95(7):708–716
van Heerebeek L et al (2006) Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113(16):1966–1973
Nguyen TT et al (1996) Maximal actomyosin ATPase activity and in vitro myosin motility are unaltered in human mitral regurgitation heart failure. Circ Res 79(2):222–226
Alpert NR, Gordon MS (1962) Myofibrillar adenosine triphosphatase activity in congestive heart failure. Am J Physiol 202:940–946
Belin RJ et al (2006) Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol 291(5):H2344–H2353
Fan D, Wannenburg T, de Tombe PP (1997) Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation 95(9):2312–2317
Bristow MR et al (1993) Reduced beta 1 receptor messenger RNA abundance in the failing human heart. J Clin Invest 92(6):2737–2745
Daaka Y, Luttrell LM, Lefkowitz RJ (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390(6655):88–91
Messer AE, Jacques AM, Marston SB (2007) Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol 42(1):247–259
van der Velden J et al (2006) Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res 69(4):876–887
Endoh M, Hori M (2006) Acute heart failure: inotropic agents and their clinical uses. Expert Opin Pharmacother 7(16):2179–2202
Parissis JT et al (2006) Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 92(12):1768–1772
Follath F et al (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360(9328):196–202
Moiseyev VS et al (2002) Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 23(18):1422–1432
Fentzke RC et al (1999) Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517(1):143–157
Chang AN et al (2005) Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in alpha-tropomyosin. J Biol Chem 280(40):34343–34349
Lang R et al (2002) Functional analysis of a troponin I (R145G) mutation associated with familial hypertrophic cardiomyopathy. J Biol Chem 277(14):11670–11678
Michele DE et al (2002) Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent, and improved by beta-blockade. Circ Res 92:255–262
Gregorevic P et al (2006) rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med 12(7):787–789
Gregorevic P et al (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10(8):828–834
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Day, S.M., Westfall, M.V. & Metzger, J.M. Tuning cardiac performance in ischemic heart disease and failure by modulating myofilament function. J Mol Med 85, 911–921 (2007). https://doi.org/10.1007/s00109-007-0181-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-007-0181-6