Abstract
Purpose
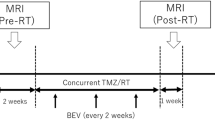
The efficacy of concomitant chemoradiation in patients with glioblastomas (GBMs) cannot be reliably assessed until several weeks after therapy completion. Our aim was to evaluate dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) as an early predictive assay for the progression-free-survival.
Methods and Materials
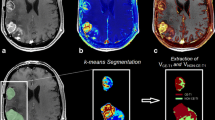
A total of 22 patients with primary GBMs underwent DCE-MRI before, during and after completion of adjuvant chemoradiation. K trans (transfer constant between the intravascular and extravascular, extracellular space), v e (extracellular, extravascular volume) and IAUGC (initial area under the gadolinium concentration time curve) and their changes into treatment were assessed as prognostic markers (12 months of progression-free-survival (PFS)).
Results
Both responders (7 subjects) and non-responders (15 subjects) experienced a reduction in the baseline IAUGC and v e values during the early phase of the treatment. This reduction was more prominent in the responders and was statistically significant for the v e (P = 0.04). Baseline K trans values among responders demonstrated statistically significant reduction during the early phase of treatment (P = 0.001). Multivariate Cox regression analysis demonstrated significant relationship between response and the early changes in K trans values during the treatment (P = 0.04). Trend to significant prognostic value demonstrated the baseline K trans, v e and IAUGC as well as the changes of IAUGC and K trans upon therapy completion.
Conclusions
Early perfusion changes during concomitant chemoradiation in GBMs can be detected by means of DCE-MRI and have significant prognostic value for the 12-month PFS.

Similar content being viewed by others
References
Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006;239(3):632–49. doi:10.1148/radiol.2393042031.
Galban CJ, Chenevert TL, Meyer CR, Tsien C, Lawrence TS, Hamstra DA, Junck L, Sundgren PC, Johnson TD, Galban S, Sebolt-Leopold JS, Rehemtulla A, Ross BD. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17(14):4751–60. doi:10.1158/1078-0432.CCR-10-2098.
Cao Y, Tsien CI, Nagesh V, Junck L, Ten Haken R, Ross BD, Chenevert TL, Lawrence TS. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int J Radiat Oncol Biol Phys. 2006;64(3):876–85. doi:10.1016/j.ijrobp.2005.09.001.
Sawlani RN, Raizer J, Horowitz SW, Shin W, Grimm SA, Chandler JP, Levy R, Getch C, Carroll TJ. Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging–pilot study. Radiology. 2010;255(2):622–8. doi:10.1148/radiol.10091341.
Voglein J, Tuttenberg J, Weimer M, Gerigk L, Kauczor HU, Essig M, Weber MA. Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol. 2011;46(6):390–400. doi:10.1097/RLI.0b013e31820e1511.
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–300. doi:10.1158/0008-5472.CAN-09-0814.
Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–7. doi:10.1158/0008-5472.CAN-11–2464.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:10.1056/NEJMoa043330.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM () Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. doi:10.1200/JCO.2009.26.3541.
Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17(2):357–67.
Orton MR, d’Arcy JA, Walker-Samuel S, Hawkes DJ, Atkinson D, Collins DJ, Leach MO. Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol. 2008;53(5):1225–39. doi:10.1088/0031-9155/53/5/005.
Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL, Jackson A, Watson Y, Davies K, Jayson GC. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med. 2006;56(5):993–1000. doi:10.1002/mrm.21066.
Chen KW, Huang SC, Yu DC. The effects of measurement errors in the plasma radioactivity curve on parameter estimation in positron emission tomography. Phys Med Biol. 1991;36(9):1183–200.
Leach MO, Morgan B, Tofts PS, Buckley DL, Huang W, Horsfield MA, Chenevert TL, Collins DJ, Jackson A, Lomas D, Whitcher B, Clarke L, Plummer R, Judson I, Jones R, Alonzi R, Brunner T, Koh DM, Murphy P, Waterton JC, Parker G, Graves MJ, Scheenen TW, Redpath TW, Orton M, Karczmar G, Huisman H, Barentsz J, Padhani A, Experimental Cancer Medicine Centres Imaging Network Steering Committee. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol. 2012;22(7):1451–64. doi:10.1007/s00330-012-2446-x.
Bankman IN. Handbook of medical imaging: processing and analysis. Academic Press series in biomedical engineering. San Diego: Academic Press; 2000.
Bastin ME, Carpenter TK, Armitage PA, Sinha S, Wardlaw JM, Whittle IR. Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. AJNR Am J Neuroradiol. 2006;27(2):402–8.
Zwick S, Brix G, Tofts PS, Strecker R, Kopp-Schneider A, Laue H, Semmler W, Kiessling F. Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. Eur Radiol. 2010;20(2):432–42. doi:10.1007/s00330-009-1556-6.
Koh TS, Bisdas S, Koh DM, Thng CH. Fundamentals of tracer kinetics for dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2011;34(6):1262–76. doi:10.1002/jmri.22795.
Mills SJ, Patankar TA, Haroon HA, Baleriaux D, Swindell R, Jackson A. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27(4):853–8.
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–121. doi:10.1152/physrev.00038.2010.
Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, Price RR, Gore JC. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25(1):1–13. doi:10.1016/j.mri.2006.09.006.
Pickles MD, Lowry M, Manton DJ, Gibbs P, Turnbull LW. Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast cancer Res Treat. 2005;91(1):1–10. doi:10.1007/s10549-004-5819-2.
Evelhoch JL. Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging. 1999;10(3):254–9.
Larcombe-McDouall JB, Mattiello J, McCoy CL, Simpson NE, Seyedsadr M, Evelhoch JL. Size dependence of regional blood flow in murine tumours using deuterium magnetic resonance imaging. Int J Radiat Biol. 1991;60(1–2):109–13.
Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, Jayson GC, Judson IR, Knopp MV, Maxwell RJ, McIntyre D, Padhani AR, Price P, Rathbone R, Rustin GJ, Tofts PS, Tozer GM, Vennart W, Waterton JC, Williams SR, Workman P, Pharmacodynamic/Pharmacokinetic Technologies Advisory Committee, Drug Development Office Cancer Research UK. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer. 2005;92(9):1599–610. doi:10.1038/sj.bjc.6602550.
Jackson A, Buckley D, Parker GJM. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Medical radiology. Berlin: Springer; 2003.
Wesolowski JR, Rajdev P, Mukherji SK. Temozolomide (Temodar). AJNR Am J Neuroradiol. 2010;31(8):1383–4. doi:10.3174/ajnr.A2170.
Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34(12):2278–86. doi:10.3174/ajnr.A3634.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32.
Sourbron SP, Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR Biomed. 2013;26:1004–27. doi:10.1002/nbm.2940.
Patriarche J, Erickson B. A review of the automated detection of change in serial imaging studies of the brain. J Digit Imaging. 2004;17(3):158–74. doi:10.1007/s10278-004-1010-x.
Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Hibberts J, Peterson PM, Prados MD, North American Brain Tumor Consortium. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–70. doi:10.1215/15228517-2007-062.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bisdas, S., Smrdel, U., Bajrovic, F.F. et al. Assessment of Progression-Free-Survival in Glioblastomas by Intratreatment Dynamic Contrast-Enhanced MRI. Clin Neuroradiol 26, 39–45 (2016). https://doi.org/10.1007/s00062-014-0328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-014-0328-0