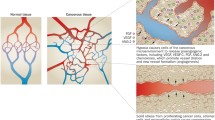
Abstract
Many therapeutic approaches to cancer affect the tumour vasculature, either indirectly or as a direct target. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has become an important means of investigating this action, both pre-clinically and in early stage clinical trials. For such trials, it is essential that the measurement process (i.e. image acquisition and analysis) can be performed effectively and with consistency among contributing centres. As the technique continues to develop in order to provide potential improvements in sensitivity and physiological relevance, there is considerable scope for between-centre variation in techniques. A workshop was convened by the Imaging Committee of the Experimental Cancer Medicine Centres (ECMC) to review the current status of DCE-MRI and to provide recommendations on how the technique can best be used for early stage trials. This review and the consequent recommendations are summarised here.
Key Points
• Tumour vascular function is key to tumour development and treatment
• Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can assess tumour vascular function
• Thus DCE-MRI with pharmacokinetic models can assess novel treatments
• Many recent developments are advancing the accuracy of and information from DCE-MRI
• Establishing common methodology across multiple centres is challenging and requires accepted guidelines

Similar content being viewed by others
References
Jackson E, Ashton E, Evelhoch JL et al (2010) Multivendor, multisite DCE-MRI phantom validation study. In: Proceedings of the 95th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA '10); December 2009; Chicago, IL, USA
Meyer CR, Armato SG, Fenimore CP et al (2009) Quantitative imaging to assess tumor response to therapy: common themes of measurement, truth data, and error sources. Transl Oncol 2:198–210
Leach MO, Brindle KM, Evelhoch JL et al (2003) Assessment of antiangiogenic and antivascular therapeutics using MRI: recommendations for appropriate methodology for clinical trials. Br J Radiol 76:S87–S91
Leach MO, Brindle KM, Evelhoch JL et al (2005) The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 92:1599–1610
Evelhoch J, Garwood N, Vigneron D et al (2005) Expanding the use of magnetic resonance in the assessment of tumor response to therapy: Workshop report. Cancer Research 65:7041–7044
Evelhoch JL (1999) Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging 10:254–259
Taylor JS, Tofts PS, Port R et al (1999) MR imaging of tumor microcirculation: promise for the new millennium. J Magn Reson Imaging 10:903–907
Tofts PS (1998) Standardisation and optimisation of magnetic resonance techniques for multicentre studies. J Neurol Neurosurg Psychiatry 64:S37–S43
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Jia G, Heverhagen JT, Polzer H et al (2006) Dynamic contrast enhanced magnetic resonance imaging as a biological marker to noninvasively assess the effect of finasteride on prostatic suburethral microcirculation. J Urol 176:2299–2304
McKeage M, Fong P, Jeffery M et al (2006) 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumours: a phase I safety study of a vascular disrupting agent. Clin Cancer Res 12:1776–1784
Head M, Jameson MB (2010) The development of the tumor vascular-disrupting agent ASA404 (vadimezan, DMXAA): current status and future opportunities. Expert Opin Investig Drugs 19:295–304
Eskens FALM, Steeghs N, Verweij J et al (2009) Phase I Dose Escalation Study of Telatinib, a Tyrosine Kinase Inhibitor of Vascular Endothelial Growth Factor Receptor 2 and 3, Platelet-Derived Growth Factor Receptor beta, and c-Kit, in Patients With Advanced or Metastatic Solid Tumours. J Clin Oncol 27:4169–4176
LoRusso PM, Gadgeel SM, Wozniak A et al (2008) Phase I clinical evaluation of ZD6126, a novel vascular-targeting agent, in patients with solid tumours. Invest New Drugs 26:159–167
Drevs J, Siegert P, Medinger M et al (2007) Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signalling inhibitor, in patients with advanced solid tumours. J Clin Oncol 25:3045–3054
Leach MO (2009) Breast cancer screening in women at high risk using MRI. NMR Biomed 22:17–27
Miyazaki K, Orton MR, Davidson RI et al (2011) The feasibility of dynamic contrast-enhanced magnetic resonance imaging to monitor and predict peptide receptor radionuclide therapy outcome in patients with neuroendocrine tumour liver metastases. Radiology 17:2012. doi:10.1148/radiol.12110770, Published online before print February
Dowell NG, Tofts PS (2007) Fast, accurate, and precise mapping of the RF field in vivo using the 180 degrees signal null. Magn Reson Med 58:622–630
Yarnykh VL (2007) Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 57:192–200
Cunningham CH, Pauly JM, Nayak KS (2006) Saturated double-angle method for rapid B1+ mapping. Magn Reson Med 55:1326–1333
Preibisch C, Deichmann R (2009) Influence of RF spoiling on the stability and accuracy of T1 mapping based on spoiled FLASH with varying flip angles. Magn Reson Med 61:125–135
Roberts C, Little R, Watson Y, Zhao S, Buckley DL, Parker GJ (2011) The effect of blood inflow and B(1)-field inhomogeneity on measurement of the arterial input function in axial 3D spoiled gradient echo dynamic contrast-enhanced MRI. Magn Reson Med 65:108–119
Buckley DL, Parker GJM (2004) T1 estimation using variable flip angle spoiled gradient echo for dynamic contrast-enhanced MRI: Arterial input measurement improves accuracy in the presence of B1 error. Proceedings of the 12th Annual Scientific Meeting of the ISMRM, Kyoto, pp 1968
Larkman DJ, Nunes RG (2007) Parallel magnetic resonance imaging. Phys Med Biol 52:R15–R55
Firbank MJ, Harrison RM, Williams ED, Coulthard A (2000) Quality assurance for MRI: practical experience. Br J Radiol 73:376–383
Ihalainen T, Sipila O, Savolainen S (2004) MRI quality control: six imagers studied using eleven unified image quality parameters. Eur Radiol 14:1859–1865
Lerski RA, de Certaines JD (1993) Performance assessment and quality control in MRI by Eurospin test objects and protocols. Magn Reson Imaging 11:817–833
Tofts P (2003) QA: Quality assurance, accuracy, precision and phantoms. In: Tofts P (ed) Quantitative MRI of the brain: measuring changes caused by disease. Wiley, Chichester, pp 55–81
Lerski RA (1993) Trial of modifications to Eurospin MRI test objects. Magn Reson Imaging 11:835–839
Jackson EF, Barboriak DP, Bidaut LM, Meyer CR (2009) Magnetic resonance assessment of response to therapy: tumor change measurement, truth data and error sources. Transl Oncol 2:211–215
Brookes JA, Redpath TW, Gilbert FJ, Murray AD, Staff RT (1999) Accuracy of T1 measurement in dynamic contrast-enhanced breast MRI using two- and three-dimensional variable flip angle fast low-angle shot. J Magn Reson Imaging 9:163–171
Preibisch C, Deichmann R (2009) T1 mapping using spoiled FLASH-EPI hybrid sequences and varying flip angles. Magn Reson Med 62:240–246
Wang J, Qiu M, Kim H, Constable RT (2006) T1 measurements incorporating flip angle calibration and correction in vivo. J Magn Reson 182:283–292
Li KL, Zhu XP, Waterton J, Jackson A (2000) Improved 3D quantitative mapping of blood volume and endothelial permeability in brain tumours. J Magn Reson Imaging 12:347–357
Parker GJ, Roberts C, Macdonald A et al (2006) Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med 56:993–1000
Bellin MF, Van Der Molen AJ (2008) Extracellular gadolinium-based contrast media: an overview. Eur J Radiol 66:160–167
Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W (2009) Risk factors for NSF: a literature review. J Magn Reson Imaging 30:1298–1308
Thomsen HS (2005) How to avoid CIN: guidelines from the European Society of Urogenital Radiology. Nephrol Dial Transplant 20:i18–22
Sam AD 2nd, Morasch MD, Collins J, Song G, Chen R, Pereles FS (2003) Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg 38:313–318
FDA (2011) Drug Safety Information for Patients and Providers. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142882.htm Accessed 15th February 2012 16.51GMT
EMEA (2011) Information on NSF http://www.ismrm.org/special/EMEA2.pdf. Accessed 15th February 2012 16.53GMT
Tofts PS, Kermode AG (1991) Measurement of the blood–brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson. Med 17:357–367
Schabel MC, Parker DL (2008) Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol 53:2345–2373
Morgan B, Utting JF, Higginson A, Thomas AL, Steward WP, Horsfield MA (2006) A simple, reproducible method for monitoring the treatment of tumours using dynamic contrast-enhanced MR imaging. Br J Cancer 94:1420–1427
Galbraith SM, Lodge MA, Taylor NJ et al (2002) Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed 15:132–142
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Orton MR, Miyazaki K, Koh DM et al (2009) Optimizing functional parameter accuracy for breath-hold DCE-MRI of liver tumours. Phys Med Biol 54:2197–2215
Henderson E, Rutt BK, Lee TY (1998) Temporal sampling requirements for the tracer kinetics modeling of breast disease. Magn Reson Imaging 16:1057–1073
Melbourne A, Atkinson D, Hawkes D (2008) Influence of organ motion and contrast enhancement on image registration. Med Image Comput Comput Assist Interv 11:948–955
Melbourne A, Atkinson D, White MJ, Collins D, Leach M, Hawkes D (2007) Registration of dynamic contrast-enhanced MRI using a progressive principal component registration (PPCR). Phys Med Biol 52:5147–5156
Buonaccorsi GA, O'Connor JP, Caunce A et al (2007) Tracer kinetic model-driven registration for dynamic contrast-enhanced MRI time-series data. Magn Reson Med 58:1010–1019
Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K (2009) Quantification of cerebral blood flow, cerebral blood volume, and blood–brain-barrier leakage with DCE-MRI. Magn Reson Med 62:205–217
Buckley DL, Shurrab AE, Cheung CM, Jones AP, Mamtora H, Kalra PA (2006) Measurement of single kidney function using dynamic contrast-enhanced MRI: comparison of two models in human subjects. J Magn Reson Imaging 24:1117–1123
Makkat S, Luypaert R, Sourbron S, Stadnik T, De Mey J (2010) Assessment of tumor blood flow in breast tumours with T1-dynamic contrast-enhanced MR imaging: impact of dose reduction and the use of a prebolus technique on diagnostic efficacy. J Magn Reson Imaging 31:556–561
Makkat S, Luypaert R, Sourbron S, Stadnik T, De Mey J (2007) Quantification of perfusion and permeability in breast tumours with a deconvolution-based analysis of second-bolus T1-DCE data. J Magn Reson Imaging 25:1159–1167
Risse F, Semmler W, Kauczor HU, Fink C (2006) Dual-bolus approach to quantitative measurement of pulmonary perfusion by contrast-enhanced MRI. J Magn Reson Imaging 24:1284–1290
Christian TF, Rettmann DW, Aletras AH et al (2004) Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology 232:677–684
Kershaw LE, Buckley DL (2006) Precision in measurements of perfusion and microvascular permeability with T1-weighted dynamic contrast-enhanced MRI. Magn Reson Med 56:986–992
Kovar DA, Lewis M, Karczmar GS (1998) A new method for imaging perfusion and contrast extraction fraction: input functions derived from reference tissues. J Magn Reson Imaging 8:1126–1134
Yang C, Karczmar GS, Medved M, Stadler WM (2004) Estimating the arterial input function using two reference tissues in dynamic contrast-enhanced MRI studies: fundamental concepts and simulations. Magn Reson Med 52:1110–1117
Yankeelov TE, Luci JJ, Lepage M et al (2005) Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging 23:519–529
Yang C, Karczmar GS, Medved M, Stadler WM (2007) Multiple reference tissue method for contrast agent arterial input function estimation. Magn Reson Med 58:1266–1275
Fan X, Haney CR, Mustafi D et al (2010) Use of a reference tissue and blood vessel to measure the arterial input function in DCEMRI. Magn Reson Med 64:1821–1826
Fluckiger JU, Schabel MC, Dibella EV (2009) Model-based blind estimation of kinetic parameters in dynamic contrast enhanced (DCE)-MRI. Magn Reson Med 62:1477–1486
Schabel MC, DiBella EV, Jensen RL, Salzman KL (2010) A model-constrained Monte Carlo method for blind arterial input function estimation in dynamic contrast-enhanced MRI: II. In vivo results. Phys Med Biol 55:4807–4823
Fritz-Hansen T, Rostrup E, Larsson HB, Sondergaard L, Ring P, Henriksen O (1996) Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med 36:225–231
Weinmann HJ, Laniado M, Mutzel W (1984) Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR 16:167–172
Horsfield MA, Thornton JS, Gill A, Jager HR, Priest AN, Morgan B (2009) A functional form for injected MRI Gd-chelate contrast agent concentration incorporating recirculation, extravasation and excretion. Phys Med Biol 54:2933–2949
Orton MR, d’Arcy JA, Walker-Samuel S et al (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 53:1225–1239
Rose CJ, Mills SJ, O'Connor JP et al (2009) Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magn Reson Med 62:488–499
Tofts PS, Steens SC, Cercignani M et al (2006) Sources of variation in multi-centre brain MTR histogram studies: body-coil transmission eliminates inter-centre differences. MAGMA 19:209–222
Balvay D, Frouin F, Calmon G et al (2005) New criteria for assessing fit quality in dynamic contrast-enhanced T1-weighted MRI for perfusion and permeability imaging. Magn Reson Med 54:868–877
Jeukens CR, van den Berg CA, Donker R et al (2006) Feasibility and measurement precision of 3D quantitative blood flow mapping of the prostate using dynamic contrast-enhanced multi-slice CT. Phys Med Biol 51:4329–4343
NCI (2004) Imaging Guidelines for Clinical Trials. http://imaging.cancer.gov/clinicaltrials/guidelines. Accessed 15th February 2012 16.57GMT
Sourbron SP, Buckley DL (2011) On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med 66:735–745
Donaldson SB, West CM, Davidson SE et al (2010) A comparison of tracer kinetic models for T1-weighted dynamic contrast-enhanced MRI: application in carcinoma of the cervix. Magn Reson Med 63:691–700
Brix G, Kiessling F, Lucht R et al (2004) Microcirculation and microvasculature in breast tumours: pharmacokinetic analysis of dynamic MR image series. Magn Reson Med 52:420–429
Johnson JA, Wilson TA (1966) A model for capillary exchange. Am J Physiol 210:1299–1303
St Lawrence KS, Lee TY (1998) An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: II. Experimental validation. J Cereb Blood Flow Metab 18:1378–1385
St Lawrence KS, Lee TY (1998) An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: I. Theoretical derivation. J Cereb Blood Flow Metab 18:1365–1377
Koh TS, Cheong LH, Hou Z, Soh YC (2003) A physiologic model of capillary-tissue exchange for dynamic contrast-enhanced imaging of tumor microcirculation. IEEE Trans Biomed Eng 50:159–167
Huang W, Li X, Morris EA et al (2008) The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumours in vivo. Proc Natl Acad Sci USA 105:17943–17948
Li X, Huang W, Morris EA et al (2008) Dynamic NMR effects in breast cancer dynamic-contrast-enhanced MRI. Proc Natl Acad Sci USA 105:17937–17942
Huang W, Tudorica LA, Li X et al (2011) Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology 261:394–403
Ewing JR, Brown SL, Lu M et al (2006) Model selection in magnetic resonance imaging measurements of vascular permeability: Gadomer in a 9 L model of rat cerebral tumor. J Cereb Blood Flow Metab 26:310–320
Naish JH, Kershaw LE, Buckley DL, Jackson A, Waterton JC, Parker GJ (2009) Modeling of contrast agent kinetics in the lung using T1-weighted dynamic contrast-enhanced MRI. Magn Reson Med 61:1507–1514
Brix G, Zwick S, Kiessling F, Griebel J (2009) Pharmacokinetic analysis of tissue microcirculation using nested models: multimodel inference and parameter identifiability. Med Phys 36:2923–2933
Buckley DL, Kershaw LE, Stanisz GJ (2008) Cellular-interstitial water exchange and its effect on the determination of contrast agent concentration in vivo: dynamic contrast-enhanced MRI of human internal obturator muscle. Magn Reson Med 60:1011–1019
Li X, Springer CS Jr, Jerosch-Herold M (2009) First-pass dynamic contrast-enhanced MRI with extravasating contrast reagent: evidence for human myocardial capillary recruitment in adenosine-induced hyperemia. NMR Biomed 22:148–157
Akaike H (1974) New Look at Statistical-Model Identification. IEEE T Automat Contr Ac 19:716–723
O'Connor JPB, Jayson GC, Jackson A et al (2007) Enhancing fraction predicts clinical outcome following first-line chemotherapy in patients with epithelial ovarian carcinoma. Clin Cancer Res 13:6130–6135
Jayson GC, Parker GJ, Mullamitha S et al (2005) Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab', leads to fluid accumulation and is associated with increased tumor vascularized volume. J Clin Oncol 23:973–981
Mills SJ, Soh C, O'Connor JP et al (2009) Tumour enhancing fraction (EnF) in glioma: relationship to tumour grade. Eur Radiol 19:1489–1498
Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE (2008) Guidelines for reporting an fMRI study. Neuroimage 40:409–414
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332
Moher D, Hopewell S, Schulz KF et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340:c869
Gaehtgens P (1980) Flow of blood through narrow capillaries: rheological mechanisms determining capillary hematocrit and apparent viscosity. Biorheology 17:183–189
Brix G, Griebel J, Kiessling F, Wenz F (2010) Tracer kinetic modelling of tumour angiogenesis based on dynamic contrast-enhanced CT and MRI measurements. Eur J Nucl Med Mol Imaging 37:S30–S51
Acknowledgements
We are grateful for comments received from Y. Hwang, E. Jackson, R. Sims, J. Evelhoch, M. Silva, W. Vennart, M. Clemence, B. Kiefer, and M. Rosen. We would also like to acknowledge the Experimental Cancer Medicine Centre (ECMC) Imaging Steering Committee (M. Leach, E. Aboagye, F. Gilbert, V. Goh, A. Jackson, D. Lomas, B. Morgan, and R. Plummer) and the ECMC Secretariat for supporting the workshop on tumour vascularity in May 2010 and coordinating activities, and all of the speakers and delegates who contributed to the meeting. The Experimental Cancer Medicine Centre Initiative is jointly funded by Cancer Research UK, the National Institute for Health Research in England, and the Departments of Health for Scotland, Wales, and Northern Ireland.
Conflict of Interest
M.O. Leach and D.J. Collins: Software developed at the Institute of Cancer Research and using some of the approaches referred to in this paper may be commercialised by the Institute of Cancer Research and commercialised or licensed to users. In those cases employees may receive some income under the Institute's rewards to inventors scheme.
B. Whitcher: Employed by GlaxoSmithKline (pharmaceutical industry) at the time of the ECMC workshop and until 6 May 2011. Now employed by Mango Solutions (software industry) and provides medical image analysis services to the pharmaceutical industry.
G. Parker: Received research grant income and consultancy income from pharmaceutical companies for work in DCE-MRI associated with clinical trials. Specifically, within the last year received research grant income from AstraZeneca, Pfiizer, GSK, Roche, Genentech, Bayer, and Merck-Serono. Received consultancy income from Bayer. Also a shareholder and director of Bioxydyn limited, a specialist imaging company with an interest in DCE-MRI.
G. Karczmar: Research has been supported by Philips Medical Systems and Bayer.
A. Padhani: Consultancy work for IXICO and Roche. DCE trial work with Roche and Oxigene.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Leach, M.O., Morgan, B., Tofts, P.S. et al. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol 22, 1451–1464 (2012). https://doi.org/10.1007/s00330-012-2446-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2446-x