Abstract
Purpose
The purpose of the study was to evaluate Response Assessment in Neuro-Oncology (RANO) criteria in glioblastoma multiforme (GBM), with respect to the Macdonald criteria and changes in contrast-enhancement (CE) volume. Related variations in relative cerebral blood volume (rCBV) were investigated.
Methods
Forty-three patients diagnosed between 2006 and 2010 were included. All underwent surgical resection, followed by temozolomide-based chemoradiation. MR images were retrospectively reviewed. Times to progression (TTPs) according to RANO criteria, Macdonald criteria and increased CE volume (CE-3D) were compared, and the percentage change in the 75th percentile of rCBV (rCBV75) was evaluated.
Results
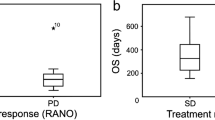
After a median follow-up of 22.7 months, a total of 39 patients had progressed according to RANO criteria, 32 according to CE-3D, and 42 according to Macdonald. Median TTPs were 6.4, 9.3, and 6.6 months, respectively. Overall agreement was 79.07% between RANO and CE-3D and 93.02% between RANO and Macdonald. The mean percentage change in rCBV75 at RANO progression onset was over 73% in 87.5% of patients.
Conclusions
In conclusion, our findings suggest that CE-3D criterion is not yet suitable to assess progression in routine clinical practice. Indeed, the accurate threshold is still not well defined. To date, in our opinion, early detection of disease progression by RANO combined with advanced MRI imaging techniques like MRI perfusion and diffusion remains the best way to assess disease progression. Further investigations that would examine the impact of treatment modifications after progression determined by different criteria on overall survival would be of great value.



Similar content being viewed by others
Abbreviations
- RANO:
-
Response Assessment in Neuro-Oncology
- GBM:
-
Glioblastoma multiforme
- CE:
-
Contrast enhancement
- rCBV:
-
Relative cerebral blood volume
- rCBV75:
-
The 75th percentile of rCBV
- TTP:
-
Time to progression
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- RECIST:
-
Response Evaluation Criteria in Solid Tumours
- FLAIR:
-
Fluid-attenuated inversion recovery
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- DSC:
-
Dynamic susceptibility contrast
- RT:
-
Radiation therapy
- GTR:
-
Gross total resection
- NTR:
-
Near total resection
- STR:
-
Subtotal resection
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- PET:
-
Positron emission tomography
References
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Tejada S, Díez-Valle R, Aldave G et al (2014) Factors associated with a higher rate of distant failure after primary treatment for glioblastoma. J Neuro-Oncol 116(1):169–175
Macdonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumours. JNCI J Natl Cancer Inst 92(3):205–216
Chinot OL, Macdonald DR, Abrey LE et al (2013) Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep 13(5):347
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Kazda T, Hardie JG, Pafundi DH et al (2015) Evaluation of RANO response criteria compared to clinician evaluation in WHO grade III anaplastic astrocytoma: implications for clinical trial reporting and patterns of failure. J Neuro-Oncol 122(1):197–203
Hu LS, Eschbacher JM, Heiserman JE et al (2012) Reevaluating the imaging definition of tumour progression: perfusion MRI quantifies recurrent glioblastoma tumour fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-Oncology 14(7):919–930
Padhani AR, Ollivier L (2001) The RECIST (Response Evaluation Criteria in Solid Tumours) criteria: implications for diagnostic radiologists. Br J Radiol 74(887):983–986
Cao Y, Tsien CI, Nagesh V et al (2006) Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int J Radiat Oncol Biol Phys 64:876–885
Sisyphe-neuroimaging software toolbox (2008). In: Proceedings of ESMRMB Congress, Valencia, Spain, Abstract 991
Wang MY, Cheng JL, Han YH, Li YL et al (2012) Measurement of tumour size in adult glioblastoma: classical cross-sectional criteria on 2D MRI or volumetric criteria on high resolution 3D MRI? Eur J Radiol 81(9):2370–2374
Henson JW, Ulmer S, Harris GJ (2008) Brain tumour imaging in clinical trials. AJNR Am J Neuroradiol 29(3):419–424
Iliadis G, Selviaridis P, Kalogera-Fountzila A et al (2009) The importance of tumour volume in the prognosis of patients with glioblastoma: comparison of computerized volumetry and geometric models. Strahlenther Onkol 185(11):743–750
van den Bent MJ, Vogelbaum MA, Wen PY et al (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s criteria. J Clin Oncol 27(18):2905–2908
Leimgruber A, Ostermann S, Yeon EJ et al (2006) Perfusion and diffusion MRI of glioblastoma progression in a four-year prospective temozolomide clinical trial. Int J Radiat Oncol Biol Phys 64(3):869–875
Khalifa J et al (2016) Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol 130(1):181–192
Kanaly CW, Mehta AI, Ding D et al (2014) A novel, reproducible, and objective method for volumetric magnetic resonance imaging assessment of enhancing glioblastoma. J Neurosurg 121(3):536–542
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26(13):2192–2197
Nowosielski M, Wiestler B, Goebel G et al (2014) Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology 82(19):1684–1692
Huang RY, Rahman R, Ballman KV et al (2017) The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res 22(3):575–581
Watling CJ, Lee DH, Macdonald DR et al (1994) Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol 12(9):1886–1889
Finn MA, Blumenthal DT, Salzman KL, et al (2007) Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 67(3):246–250
Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumour imaging. Curr Neurol Neurosci Rep 9(3):241–246
Norden AD, Drappatz J, Wen PY (2010) Malignant gliomas in adults. Blue Books Neurol 36:99–120
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53(4):291–302
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11(3):336–344
Tsien C, Galbán CJ, Chenevert TL et al (2010) Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol 28(13):2293–2299
Hu LS, Baxter LC, Smith KA et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion. AJNR Am J Neuroradiol 30(3):552–558
Sawlani RN, Raizer J, Horowitz SW et al (2010) Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging—pilot study. Radiology 255(2):622–628
Vogelbaum MA, Jost S, Aghi MK et al (2012) Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 70(1):234–243 discussion 243-244
Gahrmann R, van den Bent M, van der Holt B et al (2017) Comparison of 2D (RANO) and volumetric methods for assessment of recurrent glioblastoma treated with bevacizumab-a report from the BELOB trial. Neuro-Oncology 19(6):853–861
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a grant from the Research Innovation Therapeutics Cancerology (RITC) Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
EC-JM and VL are co-principal investigators and joint last authors.
Electronic supplementary material
Supplementary table 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Tensaouti, F., Khalifa, J., Lusque, A. et al. Response Assessment in Neuro-Oncology criteria, contrast enhancement and perfusion MRI for assessing progression in glioblastoma. Neuroradiology 59, 1013–1020 (2017). https://doi.org/10.1007/s00234-017-1899-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1899-7