Abstract
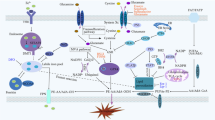
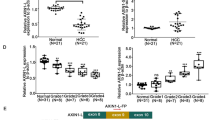
Emerging evidence suggests that ferroptosis is involved in the pathogenesis of ulcerative colitis (UC). However, the key regulator of this process remains uncertain. In this study, we aimed to explore the roles of solute carrier (SLC) family 6 member 14 (SLC6A14) in regulating ferroptosis in UC. The expression of SLC6A14 was significantly increased and positively associated with that of prostaglandin-endoperoxide synthase 2 (PTGS2) in tissue samples from patients with UC. Moreover, a series of in vitro and in vivo experiments showed that SLC6A14 knockdown markedly suppressed ferroptosis. RNA sequencing revealed that SLC6A14 inhibited the expression of P21 (RAC1)-activated kinase 6 (PAK6) and that PAK6 knockdown abolished the effects of SLC6A14 on RAS-selective lethal 3 (RSL3)-induced ferroptosis in Caco-2 cells. Furthermore, chromatin immunoprecipitation (ChIP) and Western blot analysis demonstrated that SLC6A14 negatively regulated PAK6 expression in a CCAAT enhancer binding protein beta (C/EBPβ)-dependent manner. Collectively, these findings indicate that SLC6A14 facilitates ferroptosis in UC by promoting C/EBPβ expression and binding activity to inhibit PAK6 expression, suggesting that targeting SLC6A14-C/EBPβ-PAK6 axis-mediated ferroptosis may be a promising therapeutic alternative for UC.






Similar content being viewed by others
Data availability
The microarray datasets analysed during the current study are available in the GEO (GSE134025) repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE134025.
References
Maennich D (2020) Ulcerative colitis. Nat Rev Dis Primers. https://doi.org/10.1038/s41572-020-00215-4
Ungaro R et al (2017) Ulcerative colitis. Lancet 389(10080):1756–1770. https://doi.org/10.1016/s0140-6736(16)32126-2
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Stockwell BR et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285. https://doi.org/10.1016/j.cell.2017.09.021
Mayr L et al (2020) Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat Commun 11(1):1775. https://doi.org/10.1038/s41467-020-15646-6
Xu M et al (2020) Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis 11(2):86. https://doi.org/10.1038/s41419-020-2299-1
Chen Y et al (2021) Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur J Pharmacol 911:174518. https://doi.org/10.1016/j.ejphar.2021.174518
Xu J et al (2021) Ferrostatin-1 alleviated TNBS induced colitis via the inhibition of ferroptosis. Biochem Biophys Res Commun 573:48–54. https://doi.org/10.1016/j.bbrc.2021.08.018
Chen Y et al (2020) Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett 225:9–15. https://doi.org/10.1016/j.imlet.2020.06.005
Koppula P, Zhuang L, Gan B (2021) Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12(8):599–620. https://doi.org/10.1007/s13238-020-00789-5
Ma L et al (2021) Targeting SLC3A2 subunit of system XC(−) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med 168:25–43. https://doi.org/10.1016/j.freeradbiomed.2021.03.023
Zhang Z et al (2020) The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol 36:101619. https://doi.org/10.1016/j.redox.2020.101619
Ruffin M et al (2020) Update on SLC6A14 in lung and gastrointestinal physiology and physiopathology: focus on cystic fibrosis. Cell Mol Life Sci 77(17):3311–3323. https://doi.org/10.1007/s00018-020-03487-x
Pabla BS, Schwartz DA (2020) Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin N Am 49(4):671–688. https://doi.org/10.1016/j.gtc.2020.08.003
Wirtz S et al (2017) Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12(7):1295–1309. https://doi.org/10.1038/nprot.2017.044
Camuesco D et al (2004) The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol 143(7):908–918. https://doi.org/10.1038/sj.bjp.0705941
Obermeier F et al (1999) Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol 116(2):238–245. https://doi.org/10.1046/j.1365-2249.1999.00878.x
Specht E et al (2015) Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 67(3):368–377. https://doi.org/10.1111/his.12662
Bardou P et al (2014) jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 15(1):293. https://doi.org/10.1186/1471-2105-15-293
Xie Y et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379. https://doi.org/10.1038/cdd.2015.158
Li T et al (2020) Mitochondrial PAK6 inhibits prostate cancer cell apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics 10(6):2571–2586. https://doi.org/10.7150/thno.42874
Lin H et al (2020) The miR-185/PAK6 axis predicts therapy response and regulates survival of drug-resistant leukemic stem cells in CML. Blood 136(5):596–609. https://doi.org/10.1182/blood.2019003636
Wang S et al (2020) Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci 259:118356. https://doi.org/10.1016/j.lfs.2020.118356
Bröer S, Gether U (2012) The solute carrier 6 family of transporters. Br J Pharmacol 167(2):256–278. https://doi.org/10.1111/j.1476-5381.2012.01975.x
Sikder MOF et al (2017) The Na(+)/Cl(−)-coupled, broad-specific, amino acid transporter SLC6A14 (ATB(0,+)): emerging roles in multiple diseases and therapeutic potential for treatment and diagnosis. AAPS J 20(1):12. https://doi.org/10.1208/s12248-017-0164-7
Flach CF et al (2006) Detection of elafin as a candidate biomarker for ulcerative colitis by whole-genome microarray screening. Inflamm Bowel Dis 12(9):837–842. https://doi.org/10.1097/01.mib.0000232469.23574.11
Basit F et al (2017) Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis 8(3):e2716. https://doi.org/10.1038/cddis.2017.133
Wei S et al (2020) Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater 384:121390. https://doi.org/10.1016/j.jhazmat.2019.121390
Wang H et al (2020) Mitochondria regulation in ferroptosis. Eur J Cell Biol 99(1):151058. https://doi.org/10.1016/j.ejcb.2019.151058
Yang Q et al (2020) PAK6 promotes cervical cancer progression through activation of the Wnt/beta-catenin signaling pathway. Oncol Lett 20(3):2387–2395. https://doi.org/10.3892/ol.2020.11797
Schrantz N et al (2004) Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. J Biol Chem 279(3):1922–1931. https://doi.org/10.1074/jbc.M311145200
Chen X et al (2021) Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18(5):280–296. https://doi.org/10.1038/s41571-020-00462-0
Ndoja A et al (2020) Ubiquitin ligase COP1 suppresses neuroinflammation by degrading c/EBPbeta in microglia. Cell 182(5):1156-1169.e12. https://doi.org/10.1016/j.cell.2020.07.011
Worm J et al (2009) Silencing of microRNA-155 in mice during acute inflammatory response leads to derepression of c/ebp Beta and down-regulation of G-CSF. Nucleic Acids Res 37(17):5784–5792. https://doi.org/10.1093/nar/gkp577
Guo L, Li X, Tang QQ (2015) Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem 290(2):755–761. https://doi.org/10.1074/jbc.R114.619957
Galleggiante V et al (2019) Quercetin-induced miR-369-3p suppresses chronic inflammatory response targeting C/EBP-β. Mol Nutr Food Res 63(19):e1801390. https://doi.org/10.1002/mnfr.201801390
Degagne E et al (2012) P2Y(2) receptor expression is regulated by C/EBPbeta during inflammation in intestinal epithelial cells. FEBS J 279(16):2957–2965. https://doi.org/10.1111/j.1742-4658.2012.08676.x
Lu J et al (2013) Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein beta-mediated inflammatory response and oxidative stress. J Immunol 190(7):3466–3479. https://doi.org/10.4049/jimmunol.1202862
Funding
This work was supported by The National Natural Science Foundation of China [Grant numbers 82073156, 81802843]; The Natural Science Foundation of the Jiangsu Higher Education Institutions of China [Grant number 20KJA310005]; Key Project of Medical Research of Jiangsu Commission of Health [Grant number ZDA2020008]; and Graduate Research & Practice Innovation Program of Jiangsu Province [Grant number KYCX21_2970].
Author information
Authors and Affiliations
Contributions
YJC, TGS, and WCC contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YJC, WYY, JYW, YQC, JHZ, HYJ, HYW, GBZ, TGS, QHX, and SHZ. The first draft of the manuscript was written by YJC and TGS, and all authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All animal procedures were performed in line with the principles of the Ethics Committee of Soochow University (reference number: SUDA20210918A01). All studies for human tissue samples complied the Ethics Committee of the First Affiliated Hospital of Soochow University (reference number: 2021-325).
Consent to participate
Written informed consent was obtained from the parents.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1B, 4B, and S1B.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Yan, W., Chen, Y. et al. SLC6A14 facilitates epithelial cell ferroptosis via the C/EBPβ-PAK6 axis in ulcerative colitis. Cell. Mol. Life Sci. 79, 563 (2022). https://doi.org/10.1007/s00018-022-04594-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04594-7