Abstract
Multiple sclerosis (MS) is an inflammatory, demyelinating and neurodegenerative disease of the central nervous system with unknown etiology. Currently approved disease-modifying treatment modalities are immunomodulatory or immunosuppressive. While the applied drugs reduce the frequency and severity of the attacks, their efficacy to regenerate myelin membranes and to halt disease progression is limited. To achieve such therapeutic aims, understanding biological mechanisms of remyelination and identifying factors that interfere with remyelination in MS can give respective directions. Such a perspective is given by the emerging functional profile of galectins. They form a family of tissue lectins, which are potent effectors in processes as diverse as adhesion, apoptosis, immune mediator release or migration. This review focuses on endogenous and exogenous roles of galectins in glial cells such as oligodendrocytes, astrocytes and microglia in the context of de- and (re)myelination and its dysregulation in MS. Evidence is arising for a cooperation among family members so that timed expression and/or secretion of galectins-1, -3 and -4 result in modifying developmental myelination, (neuro)inflammatory processes, de- and remyelination. Dissecting the mechanisms that underlie the distinct activities of galectins and identifying galectins as target or tool to modulate remyelination have the potential to contribute to the development of novel therapeutic strategies for MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a heterogeneous inflammatory, demyelinating and neurodegenerative disease of the central nervous system (CNS) that affects 2.5 million people worldwide. The most common clinical form is relapsing-remitting MS (RR-MS, 85%). Patients endure phases of increasing neurological deficits followed by recovery periods. After some time, approximately 60% of the patients enter a phase that is characterized by a steady decline of neurological functions with or without relapse (secondary progressive MS, SP-MS). Neuronal loss and disease progression are irreversible at this phase. Primary progressive MS (PP-MS) affects a subset of patients (10–15%) that is characterized by continuous progression of the disease from its onset. Current treatments are disease-modifying therapies and encompass application of immunosuppressive or immunomodulating drugs that reduce the number and severity of relapses in RR-MS, but these interventions are ineffective in halting disease progression [1,2,3]. Hence, there is an obvious need to develop new therapeutic strategies for progressive MS.
Remyelination following demyelination is essential for axonal survival and restoration of saltatory conduction [4,5,6,7,8], and its failure is a major cause of the neurological deficits in MS [9,10,11,12]. Therefore, restoring remyelination could prove to be an effective treatment in reversing disability and halting disease progression. Toward the aim of designing effective therapies that induce remyelination, it is important to understand the biological mechanisms that underlie the remyelination process and to identify factors that prevent remyelination in MS. Remyelination fails despite the presence of oligodendrocyte progenitor cells (OPCs) in most lesions [13,14,15,16,17,18,19]. This observation implies that either stimulating extrinsic or intrinsic factors are absent or that inhibitory signals are dominant [20,21,22,23]. In principle, this reasoning prompts to examine receptor-driven pathways and routes of communication between cells.
In terms of a recognition process of broad relevance, the abundance of glycoconjugates in the nervous system directs attention to considering the glycan part of glycolipids and glycoproteins as versatile ligand (for introduction into these glycan structures, please see [24,25,26,27,28,29,30,31]). In fact, the concept of the sugar code assigns an unsurpassed ability to store information to these glycans. Tissue receptors (lectins) are present that will ‘read’ these sugar-encoded signals, followed by ‘translation’ into effects, eliciting a broad variety of post-binding activities [32,33,34]. This functional pairing does not only depend on the complementarity of the direct ligand(glycan)–receptor(lectin) contact but also on topological parameters to achieve the inherently high levels of selectivity and specificity, letting only certain glycoconjugates with distinct (cognate) glycan display become counterreceptors for a tissue lectin [35, 36]. Following this reasoning, that is, a function of this interplay in “establishment of the cell–cell contacts and possibly also as mediators of communication between the surface and the interior of the cell”, and the abundance of glycoconjugates in the nervous system, extracts of the electric organ tissue of Electrophorus electricus proved to be the source of a lectin specific for β-galactosides that became the first member of the ga(lactose-binding)lectin family [37].
These galectins are special to exert activities inside and outside of cells by glycan- and via protein-dependent binding so that they are multifunctional [38,39,40,41,42,43,44,45]. Targeting their counterreceptors, forming molecular bridges between them in adhesion (between cells) or lattice establishment (on the membranes’ surface) and hereby triggering signaling fulfills criteria for being a versatile effector. Proceeding from work on individual galectins to a network analysis is teaching the lesson that they can be expressed at the same sites and can functionally cooperate [46, 47]. Thus, their study is a step to give meaning to the expression of certain glycans at distinct sites and to aberrations of the glycome related to the disease [48]. With focus on (re)myelination and the (immuno)pathophysiology of MS, galectins have already attained the status of notable players in this context. This review first provides an introduction to this class of effectors and then describes known roles of galectins during developmental myelination, remyelination and in the course of MS. In this context, the current status of knowledge on what galectins do, particularly in modulating immune responses and behavior of CNS glial cells, i.e., oligodendrocytes, astrocytes and microglia that are relevant to (re)myelination, is summarized as well as the relevance of galectins for MS pathology. Finally, we discuss how galectins, either as targets or tools, may help to inspire the development of novel therapeutic strategies to combat remyelination failure in MS and hence to halt disease progression.
Introduction to galectins
Galectins are a family of evolutionarily conserved proteins that share β-sandwich folding and a distinct sequence signature within the carbohydrate recognition domain (CRD). Beyond binding the canonical ligand lactose/N-acetyllactosamine (Lac/LacNAc), phylogenetic diversification has led to a divergence of the carbohydrate-binding profiles, for example, studied using frontal affinity chromatography or glycan arrays [49,50,51,52]. In principle, glycans of glycoproteins such as suited N-glycan, mucin-type O-glycan or O-mannosylated chains or of glycolipids serve as contact partners. Introduction of substituents such as a sulfate group or a sialic acid can serve as a switch for ligand activity. Of note, dynamic enzymatic interconversions from a cryptic to an active site for docking, for example, by desialylation [53], or spatiotemporally regulated shifts in the glycome ensure flexibility in controlling the recognition potential swiftly. Teaming up with the ligand specificity of the galectins, protein architecture is relevant for the nature of triggered post-binding activities, as recently highlighted by the design of custom-made variants of human galectins [54, 55]. Thus, it is important to learn about galectins’ properties in this respect.
As illustrated in Fig. 1, three types of protein structures form the set of galectins in vertebrates. Notably, non-covalently associated homodimers, linker-connected heterodimers and a structural chimera of a CRD with an N-terminal tail (consisting of non-triple-helical collagen-like repeats enabling self-interaction and a sequence bearing two sites for serine phosphorylation) facilitate to bring together ligands in different constellations and topological order [56]. The chimera-type galectin-3 (Gal-3) is thus special to build aggregates of different spatial order via contacts between CRDs or the tail, which is a substrate for various proteases that shorten its length and impair aggregation [57,58,59,60,61]. In summary, galectins combine target specificity with the ability to generate molecular associations at various sites of the cell.
Overview of the classification of the three types of modular architecture of vertebrate galectins. Proto-type galectins contain a single carbohydrate recognition domain (CRD) and are able to form monomeric or homodimeric structures. Tandem-repeat-type galectins have two distinct CRDs and are covalently associated via a linker peptide with natural variation of linker length by alternative splicing; chimera-type galectin, i.e., galectin-3 harbors one CRD and a non-lectin domain which consists of an N-terminal region and nine collagen-like repeat units that are substrates for matrix metalloproteinases (MMP-2/-7/-9/-13) and PSA-mediated cleavage at different positions shown by arrows. The N-terminal region functions as a site for serine phosphorylation. Galectin-3 is monomeric in solution in the absence of a ligand and can form aggregates in contact to oligo- or polyvalent ligands via the N-terminal tail, the CRD, or both
As first described for galectin-1 [62], galectins are synthesized in the cytosol, then reaching destinations such as the nucleus, diverse binding partners in the cytoplasm or glycans on damaged vesicle surfaces [63,64,65,66]. Overall, family members such as galectins-1 and -3 can thus perform multiple activities that depend on their cellular localization regulating cell cycle, survival (via binding of bcl-2) and RNA processing [67,68,69]. In addition, despite commonly lacking a secretion signal peptide, galectins are secreted into the extracellular space and this by non-classical pathways [70, 71] that, for example, involve exosomes [72,73,74]. Once secreted, galectins bind to matrix or cell surface glycoconjugates, readily bridging suited partners to form aggregates, and this is regulated by glycan structure, density and mode of presentation [75, 76]. When then in contact with the cell surface, galectins can re-enter the cell, there handled by the trafficking machinery as elaborately as for export and involved in sorting basolateral and apical cargo in post-Golgi compartments [77, 78]. Hereby, the residence time of counterreceptors on the surface is intimately regulated, in critical dependence of the presence of cognate glycans. Underscoring the physiological potential of galectins, their presence is under strict control, and first cases have been described for an intimate spatiotemporal co-regulation of galectin/counterreceptor presentation, for example, the Gal-1/ganglioside GM1 route of communication between effector/regulatory T cells and in axon growth induction [79, 80]. This survey explains why it is likely that galectins will also be important in CNS processes.
Since galectins are also very potent regulators of (neuro)inflammation, a dysregulation of galectins is expected to be associated with several neuroinflammatory diseases. Thus, examining the hypothesis of galectins as potent regulators of developmental myelination and remyelination as well as of a role in MS pathology is of relevance.
Role of galectins in developmental CNS myelination
Regulation of developmental myelination: a major role for neurons
Oligodendrocytes are the myelinating cells of the CNS and essential for saltatory conduction and axon survival [4,5,6,7,8]. They are generated from OPCs, which arise from neural stem cells in the subventricular zone [81]. Via the influence of a complex network of attractants and repellents such as semaphorins, OPCs proliferate and migrate via three consecutive waves throughout the developing CNS [82]. In addition, OPCs need the physical interaction with the vascular endothelium to migrate to their destination [83]. When having arrived at their destination and then subjected to local, mainly neuron-derived signals, OPCs start to differentiate towards mature, post-mitotic myelinating oligodendrocytes. Notably, part of the OPCs persist in the adult brain and develop into adult OPCs, while the generation of OPCs from neural stem cells also continues into adulthood [81, 84, 85]. The differentiation phase consists of (1) establishing contact with the newly formed axon, (2) expressing myelin genes and generating myelin membranes and (3) enwrapping the axon and creating a compacted myelin sheath. OPC differentiation requires appropriate timing for its initiation and then follows stepwise stages. The course of differentiation is well studied and defined in cultured OPCs by morphology and by the appearance of stage-specific lipid and protein markers [86,87,88]. Viewing such characteristics, OPCs are bipolar and distinguished from mature oligodendrocytes by the expression of platelet-derived growth factor receptor alpha (PDGFRα), neural/glial antigen 2 (NG2), surface gangliosides that are recognized by A2B5 antibody, and transcription factor NK2 Homeobox 2 (Nkx2.2). Concerning this aspect of the proteome, oligodendrocyte lineage cells share the expression of the oligodendrocyte transcription factor 2 (Olig2) [89, 90]. Immature oligodendrocytes are in an intermediate status of differentiation, and here the expression of 2′3′-cyclic nucleotide 3′-phosphodiesterase (CNP), a myelin-specific protein, and glycosphingolipids serve as characteristics. At this stage, the cells present high levels of galactosylceramide (GalCer) and its derivative with 3′-O-sulfation, i.e., sulfatide, at their surface. However, they do not form myelin membranes yet. Mature oligodendrocytes then have multiple processes and generate myelin membranes, while maintaining high levels of sulfatide and GalCer at their surface. On the level of proteins, mature oligodendrocytes are characterized by the expression of myelin constituents, including the major myelin basic protein (MBP) and proteolipid protein (PLP) that are present in compact myelin and myelin-associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG) that are present in non-compact myelin.
Both extrinsic factors and intrinsic signaling mechanisms that can engage transcription factors control OPC differentiation. The onset of OPC differentiation at the appropriate time and place is explained by the “derepression” model [91]. Central to it, transcription factors that maintain the status are downregulated or they are relocalized by reducing extrinsic signals that constantly inhibit differentiation. This prevents premature OPC differentiation and allows for a tightly regulated timing of OPC differentiation by stimulating factors. During development, inhibitory factors for OPC differentiation are mainly axon derived. In fact, there are several means by which axons affect OPC behavior and the correct onset of OPC differentiation. For example, inhibitory axonal factors inhibit premature OPC differentiation such as Jagged-1, neural cell adhesion molecule-bearing polysialic acid (α2,8-linked sialic acids; PSA) chains [92, 93] and LINGO-1 (leucine-rich repeat Ig domain-containing Nogo-interacting protein 1) [22, 94, 95]. Besides the axonal inhibitory signals that determine differentiation onset, axons secrete trophic factors [such as PDGF, fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1)] that regulate OPC proliferation and migration [96,97,98]. Myelin formation and OPC differentiation are promoted by the release of glutamate from synaptic vesicles along axons in vitro [99,100,101]. It appears that synapses onto myelin-forming oligodendrocytes are not required for activity-dependent myelination. In contrast, myelination is regulated by non-synaptic junctions that signal through local intracellular calcium [102]. Glutamate release from active axons initiates local production of MBP in oligodendrocytes by the assembly of cholesterol-rich microdomains and induction of Fyn kinase activity [99]. In addition, an increase of frequency of Ca2+ transient activity in sheaths is correlated with sheath elongation [103]. Interestingly, a certain oligodendrocyte is able to compartmentalize signals as different processes of the cell that act independently regarding myelin induction [102]. However, multiple stages are involved in the formation of myelin and only within a brief window of opportunity will oligodendrocytes generate new myelin segments [104, 105].
Next to neuronal-derived signals, communication of astrocytes and microglia to oligodendrocytes contributes to developmental myelination and myelin maintenance [106,107,108], while being even more prominently involved in the regulation of remyelination (see in section “Role of galectins in CNS remyelination”). Also, adaptive immune cells are involved in developmental myelination. B cells migrate to the developing brain and increase OPC proliferation by the secretion of natural IgM antibodies [109]. While the molecular and cellular regulation of developmental myelination has been studied extensively, insights into the role of the glycome and of galectins in neuronal function and OPC maturation herein are being gained over a comparatively brief period. Major steps toward defining galectins as parts of the machinery driving these processes are presented in Table 1.
Galectins in neuronal function
Initial evidence for galectin presence in neurons by haemagglutination assays [110,111,112] led to immunohistochemical localization [113, 114] and application of a galectin as tool for detecting accessible binding sites [115]. Intriguingly, lactoseries glycoconjugates appear available so that a functional pairing was hypothesized within the concept of the sugar code already at that time [116]. In this context, maturation of neurons during CNS development involves directed axonal growth towards the correct targets, accompanied by neurite branching necessary for an exploration of the environment. At present, galectins-1, -3 and -4 have been shown to be instrumental in axonal development and functioning including its myelination. Galectin-1 is prominently expressed in neurons and upregulated during sensory and motor neuron development [117, 118]. Its presence guides primary olfactory and somatosensory axons and promotes neurite sprouting, both in vitro and in vivo, i.e., as shown by aberrant topography of olfactory axons in Lgal1−/− mice [117, 119,120,121]. Galectins-3 and -4 are transiently expressed during development and downregulated at the onset of myelination [122,123,124]. Galectin-4 is present in cortical and olfactory neurons [124], here required for proper axon growth and elongation [125]. In functional terms, neuronal galectin-4 sorts and organizes transport of the axonal glycoprotein neural cell adhesion molecule L1 in a sulfatide-dependent manner [125]. Galectin-4, via binding to LacNAc termini of N-glycans, ensures proper clustering of L1 on axons in membrane microdomains and spatial organization at the axonal surface [125]. As observed in polarized epithelial cells, neuronal galectin-4 stabilizes distinct membrane microdomains and organizes apical protein transport of its cargo L1 [77, 126]. Of note, in cultured hippocampal neurons, L1 binds to immobilized galectin-3 when phosphorylated at the serine residues in the N-terminal section, what in turn regulates the segregation of L1 to discrete plasma membrane domains [127]. These domains recruit membrane–actin linkers (ERMs), which destabilize actin to stimulate local axon branching. In addition, extracellular immobilized galectin-3 promotes neurite outgrowth, but—in contrast to galectin-1—has no effect on axonal guidance in vitro [127,128,129]. When appropriately clustered, L1 binds to oligodendroglial contactin (also called F3) and activates Fyn kinase, which initiates MBP-specific mRNA synthesis and myelin biogenesis in oligodendrocytes [130,131,132,133]. In addition, axons harbor discrete galectin-4-containing domains that impede the deposition of myelin by oligodendrocytes [134]. In these myelination-excluding domains, galectin-4 interacts with axonal contactin-1, which in myelinated axons is present in the non-myelinated nodes of Ranvier [134]. Interestingly, the sequestering of the nodal protein contactin-1, the expression of neuronal galectin-4, and the size of the galectin-4-containing domains are independent of the interaction with oligodendrocytes or myelin, indicating that this is an intrinsic property of neurons. Hence, endogenous galectin-4 modulates axonal formation and outgrowth and it precludes myelin deposition, while exogenous galectins-1 and -3 determine the extent and position of axon branching. Obviously, these data do not only indicate physiological significance of individual galectins, but also substantiate functional cooperation so that further exploring the galectin network, for example, following initial data on RT-PCR signals for galectins-7 and -8 [135], is an attractive endeavor.
Galectins in oligodendrocyte maturation
In addition to the role of endogenous neuronal galectin-4 as a local axonal inhibitor of myelination, secreted neuronal galectin-4 regulates the timing of OPC differentiation and therefore the onset of myelination. Non-myelinated neurons produce and secrete galectin-4, which then binds to still uncharacterized counterreceptors that transiently appear on primary processes of immature oligodendrocytes [124]. Extracellular galectin-4 binding impairs OPC differentiation and induces dedifferentiation and proliferation in a subset of cells. Both CRDs of the heterodimeric galectin that are associated by a linker of a length of physiological significance [136], and the integrity of this display as tandem-repeat-type protein are required for galectin-4-mediated inhibition of OPC differentiation [124]. This result suggests that galectin-4 may reorganize the membrane by bringing distinct glycoconjugates in close proximity exclusively at the cell surface of primary processes. Given its association with axonal contactin-1 [134] and that oligodendroglial F3/contactin-1 triggers MBP expression [130, 131, 133, 137], it is tempting to assume that one of the galectin-4-binding sites on the oligodendroglia surface may be contactin-1. At the onset of myelination, neurons cease to secrete galectin-4, which creates a permissive environment for OPC maturation and oligodendrocytes to myelinate the bare axons. What triggers the neuron to discontinue secretion of galectin-4 remains to be determined. In other cells, this process is regulated by Src family kinase-mediated phosphorylation of its C terminus [138]. Of relevance in this respect is that no myelin deficits were observed in Src−/−, Yes−/− or Lyn−/− mice at postnatal day 28 [139]. This may be due to compensatory mechanisms, or, because earlier time points were not analyzed, potentially accelerated myelination is not revealed yet. However, Src family tyrosine kinase Fyn expression in neurons and oligodendrocytes is important for myelination [140, 141], although Fyn does not appear to be involved in the timing of OPC differentiation [139]. Also, Src kinase activity is upregulated in Fyn−/− mice [142], and it is tempting to explore the role of neuronal Fyn/Src kinases in galectin-4 phosphorylation in relation to its externalization.
Oligodendrocytes endogenously express, but do not secrete, galectin-4 in vitro. In OPCs, galectin-4 is localized to the cytoplasm, and, as OPCs are polarized cells [132, 143], galectin-4 may affect trafficking of apically located glycoproteins and -lipids, as observed in enterocyte-like cells and neurons [77, 125]. This can very well include sulfatide, especially the fraction-bearing long-chain fatty acids. This galactosphingolipid, that is enriched at the oligodendroglial surface, acts as a negative regulator of myelination [144, 145], as galectin-4 does, and it is also involved in the timed trafficking of the major myelin protein PLP to the myelin membrane [146, 147]. Upon OPC differentiation, galectin-4 shifts from a cytoplasmic to a nuclear localization [124]. In the nucleus, galectin-4 regulates the expression of MBP by binding to the transcription factor Sp1 to activate p27-mediated MBP expression [148, 149]. Hence, while neuronal galectin-4 after secretion precludes OPC differentiation, oligodendroglial galectin-4 in nuclei promotes MBP expression. These observations underscore that the location of galectins matters conspicuously.
In addition to galectin-4, galectins-1 and -3 also modulate the maturation of oligodendrocytes. Galectin-3 expression, similar to that of galectin-4, decreases upon developmental myelination, the galectin-1 level instead increases upon brain development and is leveling off in the adult rat brain [123, 150], our unpublished observations). In contrast, in vitro, galectin-1 is downregulated, whereas galectin-3 is upregulated upon OPC differentiation [123]. In addition, cultured astrocytes and microglia harbor galectins-1 and -3. Although monocultures were examined, in situ hybridization studies that confirm endogenous galectin-specific mRNA levels in glial cells in vitro and in vivo are still lacking, as well as proof that these galectins are externalized by cells of the oligodendrocyte lineage will be welcome. In Lgals3−/− mice, MBP expression is downregulated, less axons are myelinated and myelin is less compact than in wild-type mice. The hypomyelination phenotype goes along with increased number of OPCs [151] and appears to be reflected by behavioral abnormalities in Lgals3−/− mice [123]. Hence, galectin-3 plays a critical role in OPC differentiation, myelin integrity and function, likely via distinct biological processes. For example, in OPCs but not in mature oligodendrocytes, the N-terminal tail of galectin-3 is cleaved by matrix metalloproteinasae 2 (MMP-2) [123], a process shown in Fig. 1. This indicates that different biological functions of endogenous galectin-3 in OPCs and mature oligodendrocytes appear likely. As already noted, MMP-dependent cleavage impairs the N-terminal tail’s capacity toward self-aggregation [152]. In addition, processing may also affect secretion: a MMP-resistant galectin-3 variant was found to be less secreted [70]. Of interest, glycoprotein cross-linking of galectin-3 is required for apical sorting of non-raft-associated proteins, whereas in galectin-3-depleted cells cargo is mistargeted to the basolateral membrane [78]. Of relevance in this respect is that the growing myelin membrane is served by a basolateral trafficking pathway [153–155]. Therefore, galectin-3 may participate in establishing oligodendrocyte polarity, its absence interfering with myelin biogenesis and compaction, as is observed in Lgals3−/− mice [123]. Similar to Lgal3−/− mice, Lgals1−/− mice have significantly less myelinated axons, particularly in smaller diameter axons, while myelin was more loosely wrapped around axons than in wild-type mice [156]. Galectins-1 and -3 do not compensate for each other, indicating that these galectins control myelin integrity and compaction via distinct mechanisms. A case of functional antagonism between them is the inhibition of galectin-1-dependent neuroblastoma growth regulation by galectin-3 [157].
In addition to their endogenous roles, when administered to cell cultures, galectins-1 and -3 interfere with OPC maturation. Exposure in vitro to a relatively low concentration of recombinant galectin-1 impairs OPC differentiation, whereas galectin-3 treatment at the equivalent concentration increases both OPC differentiation and the extent of myelin membrane formation [123, 158]. In contrast, a relatively high concentration of galectin-1, ensuring its presence as homodimer [159, 160], increases OPC differentiation [156]. Similar contrasting effects of different galectin-1 forms have been observed in peripheral nerve injury. Thus, the common homodimeric form of galectin-1 enhances degeneration of neuronal processes in a lectin-dependent manner, whereas oxidized monomeric galectin-1 that lost its capacity to bind sugar promotes axonal regeneration [161, 162]. The distinct biological functions of galectin-1 on OPC differentiation may depend, in addition to its concentration and timing, on the status of its six cysteine residue. When oxidized at these sites, galectin-1 loses its carbohydrate-binding activity [163–166]. Notably, when immature oligodendrocytes are treated with galectin-1 at a relatively low concentration, MMP activity is enhanced which may increase the extent of MMP-mediated cleavage of galectin-3, indicating the possibility for interplay between these galectins in OPC differentiation [123, 158]. Extracellular galectin-3 accelerates OPC differentiation by modulating signaling pathways that lead to changes in actin cytoskeleton dynamics [158]. More specifically, in a CRD-dependent manner, galectin-3 reduces activation of Erk1/2 and increases Akt-mediated β-catenin signaling, an inducer of a shift from polymerized to depolymerized actin. This change in the status of the actin cytoskeleton dynamics is known to drive oligodendrocyte process outgrowth and branching, what is essential to initiate myelin membrane formation [167, 168]. In addition, extracellular galectin-3 increases MBP expression, a process only partially dependent on its CRD, which emphasizes that galectin-3 modulates OPC differentiation via multiple means including protein–protein interactions via its non-lectin part within the chimeric structure [167].
Microglia and astrocytes are cellular sources of secreted galectin-3 [123]. At least in vitro, cells of the oligodendrocyte lineage do not secrete galectin-3 (our unpublished observations). The action of extracellular galectin-3 on oligodendrocytes thus appears to be paracrine rather than autocrine. During development, galectin-3 is transiently present in microglia, and conditioned medium of galectin-3-deficient microglia does not promote OPC differentiation [123]. Oligodendroglial counterreceptors for galectin-3 remain to be identified, but galectin-3-binding sites are known to be present on cell body and processes of bipolar OPCs, with increasing morphology restricted to the cell body [158]. Of relevance, when microglia galectin-3 binds to IGF receptor 1 [169], a receptor that when activated on OPCs promotes differentiation [170–172]. Therefore, galectin-3 may delay its endocytic uptake by cross-linking the IGF receptor on the oligodendroglial cell surface, thereby potentiating IGF receptor signaling that results in enhanced OPC differentiation.
Taken together, both endogenous galectins and galectin–glycan interactions at the cell surface drive oligodendrocyte maturation. Strikingly, extracellular galectins-1, -3 and -4 modulate OPC differentiation, rationalizing their potential as novel therapeutic targets and/or tools to modulate OPC differentiation in disease. However, as galectins-3 and -4 are transiently expressed during development, their roles upon CNS demyelination and successful remyelination need to be resolved to verify and/or understand the role of galectins in MS pathology (Table 1).
Role of galectins in CNS remyelination
Regulation of remyelination: a major role of microglia and astrocytes
Demyelination is the degeneration of myelin sheaths which in the healthy CNS is followed by a spontaneous regenerative response, called remyelination. This process covers the regeneration of complete, newly formed myelin sheaths that enwrap demyelinated axons to reestablish saltatory conduction, which is salient to resolve functional deficits and to prevent axonal degeneration [10, 173–175]. In rodents, remyelination requires the generation of new mature oligodendrocytes from OPCs [176]. Therefore, remyelination morphologically resembles developmental myelination. In fact, some axonal factors including NCAM with its polysialic acid chains (PSA-NCAM), galectin-4 and LINGO-1 that are involved in the regulation of developmental myelination are re-expressed upon injury [20, 22, 177–179]. An additional level of regulatory factors, mainly provided by microglia and astrocytes, is required to limit inflammation and demyelination and to clear myelin debris. More recent studies also point to a direct role of the systemic environment in efficient remyelination, i.e., both circulating TGFβ and regulatory T cells promote OPC differentiation [180, 181]. This distinct regulation of developmental myelination and remyelination is reflected in the formation of shorter and thinner myelin sheaths on remyelinated axons compared to axons that are myelinated upon development.
For successful remyelination to occur, several tightly regulated, well-timed, and distinct sequential steps as well as interplay between distinct types of glial cells and neurons are required. To study the cells and molecular factors involved in remyelination, animal models with global or focally induced demyelination have provided valuable information. Examples of these toxin-induced demyelination animal models are the cuprizone model, where regional demyelination is most prominent in the corpus callosum upon feeding cuprizone [182–185], and the focal lysolecithin model. Here, demyelination is induced by a local injection of lysolecithin in the brain or spinal cord white matter [184, 186, 187]. Importantly, lysolecithin acts also on pericytes which leads to disruption of the blood–brain barrier (BBB) [188], while in the cuprizone model the BBB remains seemingly intact with hardly any monocyte and T-cell infiltration and primarily stimulates microglia activation [189, 190]. In fact, using mice that lack both T cells and B cells (Rag-1−/− mice), it was shown that CD4+ and CD8+ T cells are required for successful remyelination upon lysolecithin-induced demyelination [191], while T cells or B cells are not essential for cuprizone-induced de- and remyelination [192]. Studies with these experimental de-and remyelination models revealed that successful remyelination depends on OPCs adjacent to the injured area to be transcriptionally activated, followed by their proliferation and migration towards the demyelinated area, and subsequent differentiation of the recruited OPCs to mature myelinating oligodendrocytes [10]. In addition, microglia and astrocytes are recruited to the lesioned area upon toxin-induced demyelination [187, 193].
Microglia, the resident immune cells of the CNS, are one of the first responders upon demyelination: they initiate an innate inflammatory response and clear myelin debris [194]. Microglia responses are very heterogeneous and complex. Different, not yet fully defined activation states exist, of which the classical pro-inflammatory and alternative regenerative phenotype are the most studied [195–198]. Transcriptomic analysis of isolated microglia at different stages upon cuprizone-induced demyelination shows a signature that supports remyelination already at the onset of demyelination involving, among others, phagocytosis of myelin debris [199]. Clearance of degenerated myelin is essential for remyelination, as myelin proteins are known to negatively influence remyelination by inhibiting OPC differentiation [200–202].
Similarly, depletion of specific microglia/macrophage phenotypes in toxin-induced demyelination models demonstrates that while the pro-inflammatory phenotype is initially required, induction of an anti-inflammatory regenerative phenotype of microglia/macrophages is essential for effective remyelination [203]. To control clearance of myelin debris and to accomplish remyelination, bilateral cross-talk between microglia with other CNS (glia) cells is of utmost importance. Microglia promote astrocytic activation [204, 205] and modulate OPC differentiation, while astrocytes instruct microglia and OPCs [106, 206, 207]. To control demyelination and to obtain remyelination, astrocytes play a dynamic and active role. They enhance the immune response by releasing cytokines and chemokines that recruit microglia to the lesion site, inhibit demyelination by releasing anti-inflammatory cytokines and regulate myelination by transiently depositing distinct extracellular matrix molecules that guide OPC proliferation, migration and differentiation [106, 107, 178, 207, 208]. Also, astrocyte ablation delays myelin debris clearance [193], what is required for remyelination to occur [200]. Also, it inhibits the regeneration of oligodendrocytes and myelination [193]. In analogy to microglia, distinct astrocyte phenotypes exist, A1 astrocytes being the harmful type and A2 astrocytes that upregulate neurotrophic factors being protective [209]. Classically (LPS) activated microglia, via the secretion of IL1-1α, TNF and C1q, are required to generate A1 astrocytes in vivo [209]. This suggests a strong interplay between microglia and astrocytes from the onset of CNS injury onwards, concomitantly with the axon-derived secreted and adhesive factors.
Interplay of astrocytes and phagocytosing cells via galectins
As galectins are known to be involved in neuroinflammation and both endogenous and exogenous galectins modulate developmental myelination, the expression and function of galectins upon demyelinating injury and subsequent remyelination have been studied both in toxin-induced animal models and in cellular processes relevant to remyelination (Table 1, Fig. 2). Galectin-4 is transiently re-expressed on axons upon cuprizone-induced demyelination ([179], Fig. 2.3). Although no functional studies on the role of galectin-4 upon demyelination and remyelination are available, it is tempting to suggest that similar to the situation in CNS development re-expressed axonal galectin-4 may be involved in the timing of remyelination preventing premature OPC differentiation upon demyelination (Fig. 2.3a) and myelin deposition (Fig. 2.3b). Remarkably, and in contrast to developmental myelination, galectin-4 resides also in the nucleus of microglia/macrophages upon cuprizone-induced myelination ([179], Fig. 2). In vitro analysis revealed that galectin-4 is not secreted by microglia and macrophages [179]. In addition, galectin-4 protein expression is upregulated in cultured alternatively activated microglia and macrophages and present in both the cytoplasm and nucleus, suggesting that it may add to their pro-regenerative properties. In addition, galectin-4 reduces cytokine secretion of anti- and pro-inflammatory cytokines, IL-10 and TNFα in T cells [210]. On the other hand, galectin-4 administration to macrophages increases the secretion of TNFα and IL-10 [211]. This indicates that in different cell types galectins are able to induce a distinct cytokine secretion signature, which may result in different pathways detrimental or supporting remyelination. Of note, context-dependent effects of galectins reflect their ability of binding to different counterreceptors in different cells, a hallmark of their functional versatility.
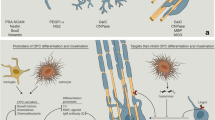
Schematic illustration of the cellular expression and role of galectins-1, -3 and -4 in the regulation of OPC differentiation upon successful remyelination. 1 Galectin-1 is mainly expressed and secreted by (reactive) astrocytes. Low galectin-1 levels (likely mainly monomeric) impair OPC differentiation (1a, [156], whereas high levels of galectin-1 (likely mainly dimeric) increase OPC differentiation (1b, [158]). Galectin-1 binds to classically activated microglia and inhibits their polarization towards a pro-inflammatory phenotype (1c, [212]), accelerates the shift towards an alternatively activated pro-myelinating microglia phenotype (1d, [156]), and increases their capacity to phagocytose remyelination-inhibiting myelin debris (1e, [156]). Via a positive feedback loop [212], galectin-1 stimulates the release of BDNF by astrocytes (1f, [217, 218]) and enhances the proliferation of neural progenitors (1g, [219]). 2Galectin-3 is expressed by microglia and oligodendrocyte lineage cells. Oligodendroglial galectin-3 is processed by MMP-2 shortening its N-terminal tail in OPCs, but not mature oligodendrocytes. Galectin-3 treatment promotes OPC differentiation (2a, [123]), may regulate astrocyte responses (2b, [221], favors polarization to pro-regenerative microglia (2c) and increases phagocytosis of myelin debris by microglia (2d, [225]). 3Galectin-4 is re-expressed by neurons and considered to be transiently released by axons to negatively regulate the differentiation of OPCs (3a, [179]). In addition, the galectin-4-containing domains on axons may impede the deposition of myelin (3b, [134]). Upon OPC differentiation, oligodendroglial galectin-4 regulates MBP promoter activity (3c, [148]). Galectin-4 is present in the nucleus and/or cytosol of microglia. The underlying mechanism(s) of action of galectins-1, -3 and -4 upon de-and remyelination is (are) summarized in Table 1
Functional studies to determine a role of exogenous galectin-1 in remyelination have been performed. Intracranial administration of galectin-1 a few days after lysolecithin-induced demyelination resulted in reduced demyelination and extensive remyelination [156]. In this model, galectin-1 accelerates the shift towards an alternatively activated pro-regenerative microglia phenotype and increases the cell’s capacity to phagocytose remyelination-inhibiting myelin debris ([156], Fig. 2.1c–e). Galectin-1 binds with increased affinity to classically activated microglia and deactivates this detrimental status by retaining the glycoprotein CD45 via lattice formation on the surface, the homodimer being ideal for cross-linking. This way, the phosphatase activity of CD45 is prolonged, which favors alternative polarization [212]. In addition, it has been suggested that galectin-1 may actively promote alternative activation of microglia by binding to neuropilin-1 (NRP-1) [213]; NRP-1 ablation in microglia fails to polarize to the anti-inflammatory phenotype [214] and galectin-1 promotes axonal regeneration upon spinal cord injury by blocking the binding of Sema3A to NRP-1/PlexinA4 complex [215]. Next to galectin-1-mediated acceleration of the shift from classical to alternative microglia polarization, galectin-1 also directly acts on cells of the oligodendrocyte lineage upon lysolecithin-induced demyelination [156], Fig. 2.1a, b). Although the underlying mechanisms remain to be explored, in analogy to neurons, galectin-1 may interfere with Sema3A binding known to prevent OPC differentiation and remyelination [216].
Bringing astrocytes into play, microglial activation is controlled by astrocytes via galectin-1 secretion. In vitro stimulation of astrocytes by anti-inflammatory signals IL-4 and TGFβ1 and galectin-1 itself led to an increase in the release of galectin-1, suggesting a positive feedback loop ([212], Fig. 2.1f). Notably, exogenously supplied galectin-1 reduces the astroglial response upon lysolecithin-induced demyelination [156]. Moreover, recombinant galectin-1 reduces astrocyte proliferation and induces their differentiation with its glycan-binding activity through the activation of protein tyrosine phosphatase [217]. This is accompanied by enhanced production of brain-derived neurotrophic factor (BDNF) [217, 218], a neuroprotective factor which is known to promote neuronal survival and neuronal development (Fig. 2.1f). Upon other types of CNS injury, galectin-1 is prominently expressed and secreted by astrocytes and enhances proliferation of neural progenitors ([219], Fig. 2.1g). Hereby, beneficial effects of exogenous galectin-1 at demyelinating conditions are established, an advantage for considering testing the lectin for a therapeutic potential.
In addition to galectin-1, galectin-3 also exerts different functions in the process of remyelination. For example, Lgals3−/− mice show a similar degree of susceptibility to cuprizone-induced demyelination as wild-type mice, but have an impaired efficiency of remyelination, as reflected by an increase in number of collapsed axons with defective myelin wraps [151]. In more detail, OPCs in cuprizone-induced demyelinated areas in Lgals3−/− mice are morphologically less complex and have a decreased ability to differentiate, likely due to the absence of exogenous galectin-3 to organize actin cytoskeletal rearrangements ([123, 167], Fig. 2.2a). In contrast, in another study, during cuprizone-induced demyelination in Lgals3−/− mice, OPC maturation is not affected by the loss of galectin-3 [220]. This may be related to a difference in the way the knock-out mice were generated. The Lgals3−/− mice that show perturbed remyelination have an inactivated galectin-3 gene that lacks an exon that encodes a part of the CRD [221, 222], while the Lgals3−/− mice that showed no effect on OPC maturation also lacked exons that are required to initiate translation and encode for the N-terminal region of galectin-3 [220, 223]. Intracranial administration of MMP-processed or full-length galectin-3 in cuprizone-fed mice may resolve whether galectin-3 is indeed beneficial for remyelination. This is conceivable, as—seen in the cuprizone model—galectin-3 expression is increased and expressed in microglial cells, but not in astrocytes, and remains high at remyelinating conditions [199], modulating their microglial phenotype ([221], Fig. 2.2c). In addition, in cuprizone-fed Lgals3−/− mice astrocytes are more hypertrophic in demyelinated lesions, also suggesting a role for (microglia) galectin-3 in regulating astroglial responses upon demyelination ([221], Fig. 2.2b). The induction of transient and focal ischemic injury in Lgals3−/− revealed that galectin-3 is indeed required for injury-induced microglial activation [169]. In contrast, neonatal Lgals3−/− mice were protected from hypoxic–ischemic brain injury [185], indicating different means of galectin-3 to modulate microglial phenotype in the adult and immature brain. Another study has demonstrated that during cuprizone-induced demyelination the presence of MMP-3 is increased and that galectin-3 is necessary to upregulate MMP-3 expression and to promote microglial activation [151]. Also, and in contrast to what is observed upon ischemic injury [169], galectin-3-deficient microglia become more proliferative upon demyelination [151]. Of importance now is to resolve whether the actions of microglia-derived galectin-3 upon demyelination are dependent on lectin binding or its non-lectin activities.
A critical part during toxin-induced demyelination is clearing the remyelination-inhibiting myelin debris by resident microglia cells [200]. Galectin-3 is involved in myelin phagocytosis mediated by the Ras/PI3K signaling pathway ([224, 225], Fig. 2.2d) and by regulation of the expression of the phagocytic receptor TREM-2b [221]. Upon demyelination in Lgals3−/−mice, TREM-2b is not detected on microglia, along with the absence of the activation marker CD68. In addition, Lgals3−/−mice were also unable to increase TNFα levels upon cuprizone treatment [221], while the mRNA levels of chemokine CCL2, a marker for classically activated microglia, remained high. Altered microglia activation in Lgals3−/−mice is also reflected by increased levels of caspase-3 activation [221], a marker for apoptosis, in microglia, indicating an anti-apoptotic role of galectin-3. While it is tempting to conclude that galectin-3 may favor polarization towards alternatively activated microglia, another study showed that the addition of galectin-3 to cultured microglia increased the expression of pro-inflammatory cytokines and enhanced the phagocytic capacities of the cells by activating the JAK-STAT cascade [226]. Also, galectin-3 is required for complete activation of TLR4 to initiate TLR4-mediated responses in microglia and for prolonging the inflammatory response [227]. This further complicates the effect of galectin-3 on microglia activation and function, suggesting that a distinct spatiotemporal course of expression of galectin-3 is required for the induction of the correct microglia phenotype to attain successful remyelination. Worth considering, posttranslational modifications such as phosphorylation and the dissection of biological functions via the non-lectin part or CRD may help understand the molecular basis for the contrasting effects of galectin-3 on microglia function.
In summary, galectins-1, -3 and -4 via their interactions act as communication cues between neurons, astrocytes, microglia and OPCs and modulate cellular responses during de- and/or remyelination (Tables 1, 2, Fig. 2). In addition, intimately regulated spatiotemporal expression and secretion of galectins are essential for regulating innate immune responses required for successful remyelination. As consequence, dysregulation in galectin action may contribute to MS pathology. This topic will be discussed next.
Galectins in MS pathology
MS pathology: a role of peripheral and resident cells
Neurological diseases that involve myelin pathology can be divided into inherited or acquired disorders (reviewed in [228, 229]). Leukodystrophies are hereditary myelin disorders that are characterized by either hypomyelination or demyelination. Strikingly, the primary affected cell type in leukodystrophies does not have to be the oligodendrocyte itself, i.e., the genetic defect may also cause dysfunction of astrocytes or microglia, emphasizing the role of other glial cells in myelin biogenesis. Next to genetic factors, viral, trauma (ischemic brain injury), toxic, metabolic and immune-mediated factors also play a role in the etiology of demyelination. MS has been known to be the archetypal acquired demyelinating disorder of the CNS. The cause of MS is unknown, although both environmental exposure and genetic susceptibility appear to play a role. MS is characterized by inflammation, demyelination, axonal damage and (astro)gliosis and manifests as demyelinated lesions at multiple regions in the brain and spinal cord [3]. Autoreactive pathogenic peripheral CD4+ helper T cells penetrate the BBB, are re-activated in brain parenchyma by CNS-associated antigen-presenting cells, and play a central role in the development of demyelinated lesions in RR-MS. The disease pathogenesis during RR-MS is driven by the fine balance between Th1 and Th17 cells, and their suppressive regulatory T cells (Tregs). These myelin-reactive peripheral cells cross the BBB and mediate myelin degeneration [230–234]. Peripheral monocyte-derived macrophages are also recruited to demyelinated lesions [235–237]. In active MS lesions, it is estimated that 55% of the macrophages arise from infiltrated monocytes [238]. In contrast, only a few peripheral macrophages are present in cuprizone-induced demyelinated lesions [190]. Infiltrated macrophages will add microglia to resolve the inflamed and demyelinated area, while differential functions are apparent, microglia being more supportive and macrophages more immune reactive [196, 239–241]. Interestingly, microglia and macrophages directly communicate with each other. This has recently been shown in a model for spinal cord injury, where infiltrated macrophages reduce microglia-mediated phagocytosis and inflammatory responses [242]. However, it is not fully understood whether the infiltration of peripheral cells is a primary autoimmune response or a secondary response to demyelination [3, 243–245], as primary degeneration of axons is also a characteristic feature of MS [246]. In fact, de-adhesion of the inner loop of myelin to the axonal surface has been postulated to be the initial event in MS lesion formation [245, 247].
Although spontaneous remyelination occurs, most commonly at early stages of MS and in active lesions, a major cause of the neurological deficits and disease progression is due to incomplete or failed remyelination, particularly at the later progressive MS stage and in chronic lesions [9, 10, 15, 239, 241, 243]. Remyelination is, however, observed in some patients at late-stage progressive MS, emphasizing the heterogeneity in MS pathology [3, 248, 249]. The factors involved in remyelination failure are many, including axonal damage, dysregulation of the cellular and molecular microenvironment within the lesions and/or failure of OPC recruitment. Strikingly, post-mortem analysis revealed that in approx. 70% of MS lesions OPCs are present [13, 15, 16], indicating that extrinsic and/or intrinsic factors in MS lesions that allow differentiation are derailed. During the active phase of an MS lesion, microglia and macrophages are skewed towards a pro-inflammatory phenotype [250, 251]. However, given the altered environmental factors in MS lesions at hand, a major subset of infiltrated macrophages and resident activated microglia acquire eventually an intermediate activation status [252, 253]. As an anti-inflammatory regenerative phenotype of microglia and macrophages is essential for effective remyelination [203], dysregulated activation of microglia and/or macrophages may contribute to remyelination failure in MS. Also, reactive astrogliosis and astrocytic scar formation negatively affect OPC recruitment and differentiation, and thereby remyelination [254], but are on the other hand also beneficial for functional CNS recovery [106, 207, 255, 256].
Dysfunction of astrocytes and/or microglia, for example, dysregulates galectin expression and secretion, disturbs their interplay, leading to a molecular environment that is non-permissive for OPC maturation. Increased expression of galectins-1, -3, -4, and -9 in CNS-resident cells is apparent in MS lesions compared to control white matter [257, 258], and galectin-1 is one of the most upregulated genes in MS-associated microglia signature [259]. Galectins are also regulators of peripheral immune responses [40, 258, 260] and given the infiltration of peripheral cells in MS lesions, galectins present in the periphery may (indirectly) contribute to remyelination failure. Indeed, next to infiltration of macrophages, infiltrating regulatory T cells have regenerative properties, by promoting OPC differentiation and remyelination [181], while Th17 cells decrease OPC differentiation and survival [261]. Therefore, before discussing whether an increased presence of these galectins in MS lesions is beneficial or detrimental to remyelination, we first describe whether these galectins, when present in the periphery, may be involved in adaptive immune responses in MS.
Galectins in MS-related neuroinflammation
Experimental models that recapitulate all aspects of MS pathology are not available, in part due to the unknown cause, if only one, and heterogeneity in MS. While in toxin-induced demyelination models pathogenic T cells are not involved in the demyelination process [191, 192], the adaptive immune system plays an important role in inducing demyelination in experimental autoimmune encephalomyelitis (EAE) models. Depending on the species, strain, and the used myelin protein/peptide, different courses, including acute, relapsing-remitting and chronic, can be initiated in EAE models [262]. The initiation and peak of the disease are mediated by Th1 and Th17 responses, while recovery from EAE is initiated by a shift towards Th2 cell responses [263], although another study found that Th2 cells also have the potential to induce EAE [264]. Furthermore, in the EAE model by controlling cytokine production and the movement of T cells, regulatory T cells have been found to be protective and mediate recovery from EAE [265, 266]. Also, as in MS lesions, infiltrated peripheral macrophages, as well as B cells, are present at the affected areas [267]. In contrast, the role of microglia in EAE is considered to be less important than in MS [243]. Therefore, the EAE model is indispensable in MS research and also exploited to elucidate the role of galectins in modulation of inflammatory response in the CNS (Table 3).
Endogenous galectin-1 expression is dynamically regulated in EAE, being increased in astrocytes at the lesion edges, and in subsets of CD4+ Th1 cells and microglia before and at the onset of EAE symptoms, while its expression remains increased in astrocytes at the chronic stage [212]. Intravenously administration of galectin-1, either before or at EAE onset, results in a reduced severity of symptoms [268], mainly by inducing tolerogenic dendritic cells, selective elimination of pro-inflammatory Th1 and Th17 cells and enhanced development of Tr1 and regulatory T cells [269, 270]. This is also shown by the inhibitory effect on T-cell proliferation upon binding of galectin-1 to NP-1, a glycoprotein counterreceptor [213, 271]. Consistently, induction of EAE in Lgals1−/− mice increases the severity of symptoms via a T helper cell response mechanism and a concomitant increase in classically activated microglia and axonal damage [270]. Moreover, adoptive transfer of galectin-1-secreting astrocytes or galectin-1-treated microglia augmented EAE symptoms via a mechanism that involves deactivation of pro-inflammatory microglia [212]. This indicates a role of this lectin as an anti-inflammatory mediator and neuroprotective agent.
Lgals3−/− mice show reduced severity upon induction of EAE [272], a sign for a detrimental role for galectin-3 in EAE pathology. Interestingly, this effect is associated with a decreased Th17 and an increased regulatory T-cell response, i.e., an underlying mechanism similar as observed for galectin-1 administration (see above), as well as decreased infiltration of peripheral macrophages [272]. In contrast, a higher incidence and more severe course of EAE is apparent in mice lacking Mgat5, an enzyme necessary for β1,6 branching (GnT-V) on N-glycans, to which galectin-3 can bind, preferably when presenting LacNAc repeats. Given the hereby caused reduction in galectin-3 counterreceptors on the T-cell surface, Mgat5−/− mice displayed enhanced T-cell receptor (TCR) clustering and diminished polarization to Th2 cells, and developed spontaneous inflammatory demyelination and neurodegeneration [273, 274]. Similarly, earlier studies have identified galectin-3 as a negative regulator of T-cell activation [273, 275]. By cross-linking TCRs and other glycoproteins on the surface of naive T cells, galectin-3 restricts TCR clustering at the site of antigen presentation, which prevents T-cell activation. Thus, the role of galectin-3 in T-cell responses in EAE is currently controversial.
Inside the CNS, galectin-3 is highly implicated in the pathophysiology of EAE. In EAE, galectin-3 is present in phagocytosing microglia and macrophages and is upregulated in areas of demyelination and myelin degeneration [276, 277]. Along with the expression of MAC-1 (CD116), which mediates myelin phagocytosis, galectin-3 (also known as MAC-2) is, as in the case for microglia, an in vivo marker for an activated phagocytosing macrophage [276, 278]. Interestingly, peripheral macrophages obtained from Lgals3−/− mice are defective to become alternatively activated [279]. Alternatively induced macrophages have increased expression and secretory activity for endogenous galectin-3 [279]. On macrophages, galectin-3 binds to CD98 and stimulates the PI3K pathway that drives the alternative activation route of macrophages [279]. Hence, this type of macrophage activation phenotype is dependent and sustained by the endogenously expressed galectin-3. In contrast, in vitro, enhanced secretion and expression of galectin-3 by classically activated human macrophages are observed [280]. Moreover, galectin-3 sustains a pro-inflammatory microglia and macrophage phenotype in a spinal cord injury model [281]. Evidently, effects of galectin-3 on phagocytosing cells are complicated, suggesting that a timed and context-dependent expression of galectin-3 is necessary for the induction of the correct microglia/macrophage phenotype. Despite the dual properties of galectin-3 in EAE, i.e., peripherally and at the lesion site, the overall impact upon demyelination in the CNS is that galectin-3 is instrumental in modulating the phenotype of macrophages at the demyelinated area.
Galectin-9, a tandem-repeat-type family member, has also been implicated in EAE development. Intraperitoneal administration of galectin-9 early after EAE induction results in a reduced severity, whereas siRNA-mediated silencing of galectin-9 results in an increased severity of clinical symptoms [282]. Galectin-9 is a binding partner for the glycoprotein Tim-3, a type-1 membrane protein specifically expressed on the surface of fully differentiated Th1 cells. Galectin-9 is a negative regulator of Th1 cell function and induces phosphorylation of Tim-3 which in turn triggers Th1 cell apoptosis, thereby shifting the balance towards Th2 cells and reducing extent of inflammation [282, 283]. Bat-3, a binding partner of intracellular tail of Tim-3, promotes proliferation and is a protective agent of Th1 cells against galectin-9-mediated cell death [284]. Also, reduced expression of Tim-3 on T cells has been suggested as an intrinsic defect that contributes to the pathogenesis of MS [285]. Blocking the interaction of galectin-9 with Tim-3 results in reduced apoptosis of T cells of RR-MS patients, but not in T cells obtained from PP-MS patients, which may relate to the upregulation of Bat-3 in PP-MS [286]. Thus, the Tim-3/Galectin-9 pathway seems to be malfunctional in PP-MS. Of note, when IFN-β, applied to treat RR-MS, is fused to galectin-9 to build a conjugate, the immunosuppressive effects of IFN-β on Th1 cells are more effective and its side effects are reduced [287]. Galectin-8 that also has the tandem-repeat-type architecture exerts similar immunosuppressive responses as galectin-9 does in EAE. Fittingly, EAE is exacerbated in Lgals8−/− mice, by modulating the balance of Th17 and Th1 cells and their Tregs [288]. This galectin induces apoptosis in Th17, but not Th1 cells, and galectin-8 administration ameliorates EAE [288]. In the clinical situation, the possibility for the occurrence of auto-antibodies against galectins should be considered (see below, [289, 290]).
In conclusion, galectins-1, -8 and -9 exert immunosuppressive and anti-inflammatory effects, and galectin-3 acts as a pro-inflammatory regulator. These observations strongly suggest that galectins-1, -3, -8 and -9 are involved in EAE/MS pathology by modulating T-cell-mediated inflammation, macrophage recruitment and function at the periphery and/or within the infiltrated lesioned areas. While the effect of galectin-4 in EAE pathology has not been examined, the ameliorating effect of sulfatide treatment on EAE (sulfatide being a counterreceptor), among others via inhibition of T-cell proliferation, is galectin-4 dependent [291]. It is obviously of importance to gain further knowledge on how these galectins play a role in remyelination failure, which mainly unfolds inside the CNS, and then how this knowledge can be used to overcome remyelination failure in MS.
Galectins in remyelination failure
Although OPCs are recruited at the demyelinated site, an environment that negatively impacts OPC differentiation is suggested to be one of major causes of remyelination failure in MS [9, 10]. Why remyelination ultimately fails in MS is still unknown and may not be assigned to one particular cause, but is likely related to multiple events in different cells and even may differ in the different type of MS lesions. MS lesions can be partly remyelinated, but remyelination is most prominent in sites of active lesions [249, 292, 293], suggesting that the molecular and cellular environment is important for remyelination efficiency. This may include a beneficial role for microglia/macrophages, when appropriately activated, and a detrimental role for the astrogliotic scar in chronic lesions [292, 294]. Given their role in remyelination, dysregulated galectin expression and/or function may contribute to remyelination failure in MS.
Galectin-1,-3, and -9 protein levels are significantly increased in MS lesions compared to control white matter [167, 258], a clear hint of the contribution to MS pathology, including remyelination failure. In MS lesions, galectin-1 is mainly localized to the cytoplasm of microglia/macrophages, while the number of astrocytes harboring galectin-1 is decreased in astrocytes compared to control white matter [258]. In contrast to cuprizone-mediated demyelination, microglia/macrophages rather than astrocytes appear as main cellular source of galectin-1 in MS. Studied by western blot analysis in vitro, MS astrocytes externalize relatively more galectin-1 as homodimers, while normal astrocytes mainly secrete monomers [258]. In addition, in contrast to normal astrocytes, galectin-1 is localized in nuclei of MS astrocytes, both in vitro and in vivo [258]. This may be a feature of A1 astrocytes, which are observed in lesions of RR-MS patients [209], while their role in remyelination (failure) remains to be established. Presence of galectin-1 as homodimers may be beneficial for OPC differentiation when ligated to galectin-1-binding sites on OPCs [156, 295], while galectin-1 monomers secreted by normal astrocytes may prevent OPC differentiation [123]. Also, galectin-1 deactivates classically activated microglia, thereby favoring their alternative activation [212] and inducing myelin phagocytic capacity, both processes beneficial for OPC differentiation [200, 203].
Galectin-3 expression is increased in active MS lesions, and the lectin is present in the cytoplasm of microglia/macrophages and astrocytes [258]. At first glance, increased galectin-3 expression seems to be beneficial for OPC differentiation, as exogenously applied galectin-3 directly promotes OPC differentiation [123], and indirectly modulates OPC differentiation by playing a pivotal role in the switch towards a regenerative phenotype of microglia/macrophages [225, 276]. Nevertheless, although more frequently observed in active lesions compared to other MS lesions, complete remyelination fails in MS lesions. This may imply that (1) galectin-3 is not secreted at the lesion site, (2) exogenous galectin-3 is not sufficient to induce OPC differentiation, i.e., other local inhibitory factors may be dominant, or (3) OPCs and/or microglia/macrophages may lack cognate determinants for galectin-3 to initiate a response.
Although not observed at the mRNA level [258], axonal galectin-4 is (re)-expressed in chronic MS lesions, as also observed in cuprizone-induced demyelination and likely a default response to demyelination [179]. Provided that axonal galectin-4 is secreted in MS lesions, galectin-4 may impair OPC differentiation [179]. Its presence in axons per se likely prevents myelination deposition [134]. In active MS lesions, galectin-4 was observed in the nucleus and cytoplasm of activated microglia/macrophages, which efficiently endocytose galectin-4 in vitro [179]. In this way, microglia/macrophages may scavenge galectin-4 away from immature oligodendrocytes and attenuate the negative role of galectin-4 on differentiation [179]. Its persistent presence in and potential secretion by demyelinated axons indicate that axonal galectin-4 in MS lesions may be a potential cause of remyelination failure in MS, particularly in chronic lesions where the number of microglia/macrophages is reduced [296, 297].
The role of galectins-8 and -9 in demyelination–remyelination models has not been thoroughly investigated yet. Although total galectin-8 protein expression is not enhanced, at the cellular level galectin-8 is abundantly present in microglia/macrophages in active MS lesions, while being absent in microglia in tissue adjacent to the lesion [258]. The presence of galectins-8 and -9 at the lesion site may have immunomodulating roles on the infiltrated T cells that are present in MS lesions, which may include selective apoptosis of Th17 and Th1 cells. Th cells and cytotoxic T cells are localized perivascularly, as well as being diffusely present throughout MS lesions [292, 298]. Galectin-9 is produced and secreted by activated astrocytes [299–301] and synergizes with the TLR3 agonist poly(I:C) to increase TNF-α secretion by microglia in a Tim-3-independent manner [299]. Interestingly, poly(I:C)-stimulated microglia enhance galectin-9 expression in astrocytes, emphasizing a role for galectin-9 in astrocyte–microglial cross-talk. Although galectin-9-specific mRNA levels are increased in astrocytes that were isolated from MS lesions [257], enhanced protein expression of galectin-9 in MS lesions is related to its presence in microglia/macrophages rather than astrocytes [258]. Remarkably, galectin-9 is mainly present in microglia/macrophage nuclei in active lesions, while in chronic active MS lesions galectin-9 exclusively localizes to the cytosol [258]. This nuclear-to-cytoplasmic relocation may reflect changes in galectin-9 function, as respective shuttling of galectin-3 is known to exert [66]. A potential role of endogenous galectin-9 in microglia/macrophages polarization and secretion of galectin-9 by microglia/macrophages remain to be determined. Notably, galectin-9 levels are higher in CSF of SP-MS patients than in CSF of RR-MS patients, which is likely a reflection of innate rather than adaptive immune responses [302]. This indicates that galectin-9 is involved in the pathophysiology of SP-MS, and given its role in interglial communication, may contribute to remyelination failure by retaining or inducing a pro-inflammatory microglia/macrophage phenotype.
In summary, galectins-1,-3, -4, -8 and -9 may contribute to remyelination failure in MS lesions. Although several questions remain open on the contribution of galectins to remyelination failure, the fact that several galectins appear to be involved in successful remyelination combined with the derailed regulation of galectins in MS lesions, investing further efforts to delineate, if possible, a therapeutic potential appears to be warranted.
Galectins: novel targets or tools to overcome remyelination failure in MS?
A favored strategy to overcome remyelination failure in MS is to promote OPC differentiation. Several compounds, including benztropine, are known to promote remyelination in vivo after cuprizone treatment by direct antagonism of receptors [303]. Although these compounds are very effective in promoting OPC differentiation (reviewed in [304]), OPCs in MS lesions usually face cellular and molecular environments that inhibit their differentiation. Given the multifaceted actions of galectins in interglial communication, there are several hurdles that have to be overcome before considering modifying therapies that are based on the gain or loss of (extracellular) galectin function.
The first is to define whether galectins may be useful to overcome remyelination failure, also taking into account the context of the cellular environment in the distinct MS lesions. Galectins-1 and -3 act as modulators of microglia and macrophage activation and phenotype, driving the onset of remyelination. In addition, galectins-1 and -3 enhance, whereas galectin-4 impairs OPC differentiation. Therefore, in active MS lesions, where microglia and macrophages are evenly distributed throughout the lesion, galectins-1 and -3 may help to skew the intermediate phenotype microglia and macrophages towards an anti-inflammatory phenotype and increase phagocytic capacity [156, 212, 279]. Microglia and macrophages are hardly present in chronic inactive MS lesions, while their presence is limited to (parts of) the lesion border of chronic active lesions [249, 292]. Therefore, targeted delivery of these galectins may promote OPC differentiation in chronic lesions. However, in chronic lesions galectin-4 is also abundantly present, in addition to many other negative regulators of OPC differentiation. Hence, it is essential to critically assess whether galectins-1 and -3 can overcome remyelination failure in a MS lesion environment, and to verify whether a specific galectin-4 antagonist such as a high-affinity sulfatide derivative or a neutralizing antibody against galectin-4 counteracts its inhibiting effect on OPC differentiation.
A second hurdle to be cleared is that the biochemical nature of the binding sites of galectins-1, -3, and 4 on microglia, macrophages, astrocytes, and cells of the oligodendrocyte lineage is not known yet. Identification of their counterreceptors, e.g., being a protein and/or a glycosphingolipid, will provide useful information about the chain of events toward effects. Equally important, although the activity profiles of galectins-1 and -3 appear to be similar, these galectins are not redundant and can likely act via different mechanisms at different sites, as they do not compensate for each other in experimental models. Therefore, it is of utmost importance to define the biochemical nature of counterreceptors for galectins, in the case of galectin-1 especially for both forms. Notably, the glycosylation signature of the putative galectin counterreceptors(s) may alter during development and in different phases of de- and remyelination, and may thus be only transiently suited for the galectin. How access to the termini of N-glycans of the α5β1-integrin is regulated via α2,6-sialylation and sialic acid biosynthesis as crucial molecular switches to enable galectin-1-dependent anoikis induction by the tumor suppressor p16INK4a as master factor provides a salient lesson for such mechanisms [305]. Indeed, glycosylation is subject to dynamic regulation, which definitely influences the extent of lattice formation by galectins [306]. Respective molecular switches with clinically relevant implications have been disclosed in the case of Tn sialylation by ST6GalNAc2 that blocks production of O-glycan core 1/2 counterreceptors for galectin-3 and hereby attenuates breast cancer metastasis [307] and in the case of prostate cancer progression, in which O-glycan core 2 presence and susceptibility to galectin-1 and sialylated core 1 presence and binding to galectin-4 let the clinical course go in opposite directions [308–310]. Also, oligodendroglial counterreceptors for galectins-3 and -4 are transiently expressed and spatially distributed at the surface [123, 124]. Identification of binding sites of galectins will also provide information whether these surface receptors are present in MS lesions and can thus be targeted by galectins when applied as a tool. As indicated above for the sialylation status of N-glycans and also known for enzymatic conversion among gangliosides, i.e., GD1a to GM1, or the shift from core 1 to core 2 O-glycans, the glycosylation status of glycoconjugates influences avidity of galectins binding, and disease-related changes in glycosylation may prevent galectin binding. Environmental and genetic factors are shown to dysregulate N-glycosylation by Golgi-localized enzymes that could lead to inflammatory demyelination and neurodegeneration in MS [311]. Finally, as the same galectin has multifaceted roles in different cells at different stages of the remyelination process, knowing the identity of the counterreceptor(s) will allow for more specific understanding of post-binding effects.
Galectins are potent modulators of peripherally immune responses and in this way may affect the clinical course of MS [268, 269, 272, 282, 288]. Hence, a third hurdle is that targeted delivery of galectins or their agonists/antagonists to MS lesions is required. For example, modulation of galectin levels may be accomplished by their local secretion at the lesion area from engineered nanocarriers and exosomes that pass the BBB. Also, it is essential to determine their effect on axons and astrocytes in the demyelinated areas. For example, galectin-1 dimers induce neuronal degeneration [162] and promote astrocyte differentiation [217]. On the other hand, the anti-inflammatory properties of galectin-1 may overcome the effect of infiltrated T cells [312, 313]. In addition, the heterogeneity and region-specific differences in microglia, astrocytes and OPCs in, for example, gray and white matter areas also need to be considered, as well as that timely expression of several galectins is crucial for proper remyelination. Hence, targeting galectin–glycan interactions may represent a new therapeutic approach for promoting remyelination in MS lesions, provided that a comprehensive analysis on their effect on all lesion-resident cells has been performed.
Having emphasized the importance of the protein architecture for activity (Fig. 1), it is noteworthy that altering protein design is a means to generate new classes of galectin-based effectors, to block or to induce activities with clinical benefit. Galectin-3, for example, can become a cross-linking homodimer like galectin-1, and galectin-1 a galectin-3-like chimera-type protein [314]. This special type of variant of galectin-1, monomeric in solution in contrast to the galectin-1 homodimer, has been shown to serve as functional antagonist of the wild-type protein [55]. The CRDs of galectins-3 and -4, here especially the N-terminal unit, may likewise serve as galectin-based means to saturate counterreceptors and hereby preclude triggering of harmful effects by the physiologically present galectin. Due to the high target specificity for glycoconjugates of each domain that they share with the wild-type proteins, this engineered type of reagent will likely be preferable to sugar-based inhibitors. Concerning galectin blocking, another means is specific (auto)antibodies, briefly referred to above.
Autoantibodies against galectins in MS
Autoantibodies against galectins may play an important role in the onset or progression of immune diseases [315, 316], and the presence of autoantibodies could contribute to a continuous immune dysregulation in MS. Furthermore, autoantibodies against galectins may be a possible therapeutic target to treat MS [288, 289, 317], while on the other hand autoantibodies may also interfere with the action of galectins when therapeutically applied. A recent study found that sera from SP-MS patients contain autoantibodies against galectin-3, which may serve as a biomarker for SP-MS [317]. Anti-galectin-3 antibody binding to galectin-3 at the surface of human brain microvascular endothelial cells increases, among others, the expression of ICAM-1 on brain endothelial cells. This contributes to the disturbance of BBB integrity, resulting in leukocyte leakage to the CNS [317]. Interestingly, fingolimod, an oral sphingosine-1-phosphate-receptor modulator used for treatment of RR-MS, leads to reduced level of infiltration of aggressive lymphocytes and was suggested to prevent endothelial cell activation induced by anti-galectin-3 antibodies [317]. Similarly, when administered at the time of EAE immunization, the FDA-approved drug for MS, glatiramer acetate (copolymer 1) reduces galectin-3 expression on macrophages at the lesion area [276]. Anti-galectin-8 antibodies have also been detected in sera and CSF of RR-MS patients: these proteins neutralize the immunosuppressive role of galectin-8 [288] and may thus worsen relapses. Hence, anti-galectin-8 antibodies may hold promise as a potential early prognostic marker. In addition, levels of autoantibodies against galectin-1 were significantly higher in sera from MS patients [289], and it would be interesting to determine whether these antibodies contribute to disease pathology.
Hence, the presence of anti-galectin autoantibodies in the clinical situation in situ introduces a further level to be considered, their presence and significance warranting further efforts. In addition, anti-galectin autoantibodies may serve as a (prognostic) biomarker of disease progression. However, their presence may be a complicating factor when targeting galectin–glycan interactions to promote remyelination in MS lesions.
Concluding remarks
Why remyelination ultimately fails in MS is still unknown. This review provides a survey of galectin involvement and reflects the multifunctionality of these tissue lectins. Indeed, galectins have multifaceted functions in modulating innate and adaptive immune responses in MS and are actively involved in regulating successful remyelination by affecting OPC differentiation in a direct and indirect manner. Galectins-1, -3 and -4 can be considered as factors that determine OPC differentiation and thereby (re)myelination by placing glial communication in the right time and spatial context. Identifying the biochemical nature of the counterreceptors and the chain of post-binding events as well as the antagonism/synergy between galectins in situ are open issues that need to be resolved. Also, a role of galectins that so far have not been investigated in the context of remyelination needs to be considered. For example, galectin-2, acting via the TLR4 pathway, skews macrophages toward a pro-inflammatory phenotype [318], which may also be valid in MS lesions. Interestingly, proto-type galectins-1, -2 and -7 induce apoptosis in activated T cells via different profiles of caspase activation [319]. In addition, involvement of members of other lectin families such as myelin-associated glycoprotein (siglec-4) could be worth exploring [320], and this on the network level considering various emerging modes of functional cooperation [321]. Altogether, the evidence and considerations presented in this review emphasize that dynamic expression, secretion and re-uptake of galectins, all in an intimately regulated manner, are crucial for remyelination to take place. Further research is definitely needed to understand the mechanisms that control the properties of galectins and their effect on OPC differentiation, astrocytes, microglia/macrophage activation, neurodegeneration and T cells. Thus, galectins do have potential as target or tool for the development of remyelination-based therapies in MS, but more work at the basic science level is required prior to attempting to realize their potential.
References
Torkildsen Ø, Myhr K-M, Bø L (2016) Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol 23(Suppl 1):18–27. https://doi.org/10.1111/ene.12883
Fogarty E, Schmitz S, Tubridy N et al (2016) Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: systematic review and network meta-analysis. Mult Scler Relat Disord 9:23–30. https://doi.org/10.1016/j.msard.2016.06.001
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2015) Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci 8:35. https://doi.org/10.3389/fnmol.2015.00035
Franklin RJM, Goldman SA (2015) Glia disease and repair—remyelination. Cold Spring Harb Perspect Biol 7:a020594. https://doi.org/10.1101/cshperspect.a020594
Fünfschilling U, Supplie LM, Mahad D et al (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. https://doi.org/10.1038/nature11007
Irvine KA, Blakemore WF (2008) Remyelination protects axons from demyelination-associated axon degeneration. Brain 131:1464–1477. https://doi.org/10.1093/brain/awn080
Lee Y, Morrison BM, Li Y et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. https://doi.org/10.1038/nature11314
Franklin RJM (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705–714. https://doi.org/10.1038/nrn917
Franklin RJM, ffrench-Constant C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855. https://doi.org/10.1038/nrn2480
Fancy SPJ, Kotter MR, Harrington EP et al (2010) Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol 225:18–23. https://doi.org/10.1016/j.expneurol.2009.12.020
Hagemeier K, Brück W, Kuhlmann T (2012) Multiple sclerosis—remyelination failure as a cause of disease progression. Histol Histopathol 27:277–287. https://doi.org/10.14670/HH-27.277
Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–173. https://doi.org/10.1056/NEJMoa010994
Goldschmidt T, Antel J, Konig FB et al (2009) Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72:1914–1921. https://doi.org/10.1212/WNL.0b013e3181a8260a
Kuhlmann T, Miron V, Cuo Q et al (2008) Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131:1749–1758. https://doi.org/10.1093/brain/awn096
Lucchinetti C, Brück W, Parisi J et al (1999) A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 122:2279–2295
Miron VE, Kuhlmann T, Antel JP (2011) Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta 1812:184–193. https://doi.org/10.1016/j.bbadis.2010.09.010
Wolswijk G (1998) Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci 18:601–609
Wolswijk G (2002) Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125:338–349
Charles P, Reynolds R, Seilhean D et al (2002) Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125:1972–1979
John GR, Shankar SL, Shafit-Zagardo B et al (2002) Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med 8:1115–1121. https://doi.org/10.1038/nm781
Mi S, Miller RH, Lee X et al (2005) LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 8:745–751. https://doi.org/10.1038/nn1460
Stoffels JMJ, de Jonge JC, Stancic M et al (2013) Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136:116–131. https://doi.org/10.1093/brain/aws313
Bhide GP, Colley KJ (2017) Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol 147:149–174. https://doi.org/10.1007/s00418-016-1520-x
Buddecke E (2009) Proteoglycans. In: Gabius H-J (ed) The sugar code. Fundamentals of glycosciences. Wiley-VCH, Weinheim, pp 199–216
Corfield A (2017) Eukaryotic protein glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 147:119–147. https://doi.org/10.1007/s00418-016-1526-4
Corfield AP, Berry M (2015) Glycan variation and evolution in the eukaryotes. Trends Biochem Sci 40:351–359. https://doi.org/10.1016/j.tibs.2015.04.004
Kopitz J (2009) Glycolipids. In: Gabius H-J (ed) The sugar code. Fundamentals of glycosciences. Wiley-VCH, Weinheim, pp 177–198
Kopitz J (2017) Lipid glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 147:175–198. https://doi.org/10.1007/s00418-016-1518-4
Reuter G, Gabius H-J (1999) Eukaryotic glycosylation: whim of nature or multipurpose tool? Cell Mol Life Sci 55:368–422. https://doi.org/10.1007/s000180050298
Schengrund C-L (2015) Gangliosides: glycosphingolipids essential for normal neural development and function. Trends Biochem Sci 40:397–406. https://doi.org/10.1016/j.tibs.2015.03.007
Gabius H-J, Roth J (2017) An introduction to the sugar code. Histochem Cell Biol 147:111–117. https://doi.org/10.1007/s00418-016-1521-9
Kaltner H, García Caballero G, Ludwig A-K et al (2018) From glycophenotyping by (plant) lectin histochemistry to defining functionality of glycans by pairing with endogenous lectins. Histochem Cell Biol 149:547–568. https://doi.org/10.1007/s00418-018-1676-7
Manning JC, Romero A, Habermann FA et al (2017) Lectins: a primer for histochemists and cell biologists. Histochem Cell Biol 147:199–222. https://doi.org/10.1007/s00418-016-1524-6
Gabius H-J, Manning JC, Kopitz J et al (2016) Sweet complementarity: the functional pairing of glycans with lectins. Cell Mol Life Sci 73:1989–2016. https://doi.org/10.1007/s00018-016-2163-8
Kaltner H, Gabius H-J (2019) Sensing glycans as biochemical messages by tissue lectins: the sugar code at work in vascular biology. Thromb Haemost 119:517–533. https://doi.org/10.1055/s-0038-1676968
Teichberg VI, Silman I, Beitsch DD, Resheff G (1975) A β-d-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci USA 72:1383–1387. https://doi.org/10.1073/pnas.72.4.1383
Cummings RD, Liu F-T (2009) Galectins. Cold Spring Harbor Laboratory Press, New York
Yang R-Y, Rabinovich GA, Liu F-T (2008) Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10:e17. https://doi.org/10.1017/S1462399408000719
Rabinovich GA, Toscano MA (2009) Turning “sweet” on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 9:338–352. https://doi.org/10.1038/nri2536
Gabius H-J (1997) Animal lectins. Eur J Biochem 243:543–576. https://doi.org/10.1111/j.1432-1033.1997.t01-1-00543.x
Barondes SH (1997) Galectins: a personal overview. Trends Glycosci Glycotechnol 9:1–7. https://doi.org/10.4052/tigg.9.1
Kasai K (1997) Galectin: intelligent glue, non-bureaucratic bureaucrat or almighty supporting actor. Trends Glycosci Glycotechnol 9:167–170. https://doi.org/10.4052/tigg.9.167
Kasai K (2018) Galectins: quadruple-faced proteins. Trends Glycosci Glycotechnol 30:SE221–SE223. https://doi.org/10.4052/tigg.1745.7se
Kaltner H, Toegel S, Garcia Caballero G et al (2017) Galectins: their network and roles in immunity/tumor growth control. Histochem Cell Biol 147:239–256. https://doi.org/10.1007/s00418-016-1522-8
Manning JC, García Caballero G, Knospe C et al (2017) Network analysis of adhesion/growth-regulatory galectins and their binding sites in adult chicken retina and choroid. J Anat 231:23–37. https://doi.org/10.1111/joa.12612
Weinmann D, Kenn M, Schmidt S et al (2018) Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with galectins-1 and -3. Cell Mol Life Sci 75:4187–4205. https://doi.org/10.1007/s00018-018-2856-2
Gabius H-J (2017) How to crack the sugar code. Folia Biol (Praha) 63:121–131
Gao C, Hanes MS, Byrd-Leotis LA et al (2019) Unique binding specificities of proteins toward isomeric asparagine-linked glycans. Cell Chem Biol 26:535.e4–547.e4. https://doi.org/10.1016/j.chembiol.2019.01.002
Hirabayashi J, Hashidate T, Arata Y et al (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta 1572:232–254
Iwaki J, Hirabayashi J (2018) Carbohydrate-binding specificity of human galectins: an overview by frontal affinity chromatography. Trends Glycosci Glycotechnol 30:SE137–SE153. https://doi.org/10.4052/tigg.1728.1se
Stowell SR, Arthur CM, Mehta P et al (2008) Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 283:10109–10123. https://doi.org/10.1074/jbc.M709545200
Ledeen RW, Kopitz J, Abad-Rodríguez J, Gabius H-J (2018) Glycan chains of gangliosides: functional ligands for tissue lectins (siglecs/galectins). Progr Mol Biol Transl Sci 156:289–324. https://doi.org/10.1016/BS.PMBTS.2017.12.004
Kopitz J, Xiao Q, Ludwig A-K et al (2017) Reaction of a programmable glycan presentation of glycodendrimersomes and cells with engineered human lectins to show the sugar functionality of the cell surface. Angew Chem Int Ed 56:14677–14681. https://doi.org/10.1002/anie.201708237
Ludwig A-K, Michalak M, Xiao Q et al (2019) Design–functionality relationships for adhesion/growth-regulatory galectins. Proc Natl Acad Sci USA 116:2837–2842. https://doi.org/10.1073/PNAS.1813515116
Leffler H, Carlsson S, Hedlund M et al (2002) Introduction to galectins. Glycoconj J 19:433–440. https://doi.org/10.1023/B:GLYC.0000014072.34840.04
Ahmad N, Gabius H-J, André S et al (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem 279:10841–10847. https://doi.org/10.1074/jbc.M312834200
Flores-Ibarra A, Vértesy S, Medrano FJ et al (2018) Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci Rep 8:9835. https://doi.org/10.1038/s41598-018-28235-x
Halimi H, Rigato A, Byrne D et al (2014) Glycan dependence of galectin-3 self-association properties. PLoS One 9:e111836. https://doi.org/10.1371/journal.pone.0111836
Hughes RC (1994) Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology 4:5–12. https://doi.org/10.1093/glycob/4.1.5
Kopitz J, Vértesy S, André S et al (2014) Human chimera-type galectin-3: defining the critical tail length for high-affinity glycoprotein/cell surface binding and functional competition with galectin-1 in neuroblastoma cell growth regulation. Biochimie 104:90–99. https://doi.org/10.1016/j.biochi.2014.05.010
Wilson TJ, Firth MN, Powell JT, Harrison FL (1989) The sequence of the mouse 14 kDa β-galactoside-binding lectin and evidence for its synthesis on free cytoplasmic ribosomes. Biochem J 261:847–852. https://doi.org/10.1042/bj2610847
Wang JL, Gray RM, Haudek KC, Patterson RJ (2004) Nucleocytoplasmic lectins. Biochim Biophys Acta 1673:75–93. https://doi.org/10.1016/j.bbagen.2004.03.013
Hong M-H, Weng I-C, Liu F-T (2018) Galectins as intracellular regulators of cellular responses through the detection of damaged endocytic vesicles. Trends Glycosci Glycotechnol 30:SE179–SE184. https://doi.org/10.4052/tigg.1733.1se
Sato S (2018) Cytosolic galectins and their release and roles as carbohydrate-binding proteins in host pathogen interaction. Trends Glycosci Glycotechnol 30:SE199–SE209. https://doi.org/10.4052/tigg.1739.1se
Haudek KC, Spronk KJ, Voss PG et al (2010) Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta 1800:181–189. https://doi.org/10.1016/j.bbagen.2009.07.005
Dagher SF, Wang JL, Patterson RJ (1995) Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA 92:1213–1217
Vyakarnam A, Dagher SF, Wang JL, Patterson RJ (1997) Evidence for a role for galectin-1 in pre-mRNA splicing. Mol Cell Biol 17:4730–4737. https://doi.org/10.1128/MCB.17.8.4730
Liu F-T, Patterson RJ, Wang JL (2002) Intracellular functions of galectins. Biochim Biophys Acta 1572:263–273. https://doi.org/10.1016/S0304-4165(02)00313-6
Nangia-Makker P, Raz T, Tait L et al (2007) Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res 67:11760–11768. https://doi.org/10.1158/0008-5472.CAN-07-3233
Hughes RC (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473:172–185
Théry C, Boussac M, Véron P et al (2001) Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166:7309–7318
Barrès C, Blanc L, Bette-Bobillo P et al (2010) Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 115:696–705. https://doi.org/10.1182/blood-2009-07-231449
Bänfer S, Schneider D, Dewes J et al (2018) Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci USA 115:E4396–E4405. https://doi.org/10.1073/pnas.1718921115
Vasta GR, Ahmed H, Bianchet MA et al (2012) Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects. Ann NY Acad Sci 1253:E14–E26. https://doi.org/10.1111/j.1749-6632.2012.06698
Xiao Q, Ludwig A-K, Romanò C et al (2018) Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc Natl Acad Sci USA 115:E2509–E2518. https://doi.org/10.1073/pnas.1720055115
Delacour D, Gouyer V, Zanetta J-P et al (2005) Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol 169:491–501. https://doi.org/10.1083/jcb.200407073
Delacour D, Greb C, Koch A et al (2007) Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic 8:379–388. https://doi.org/10.1111/j.1600-0854.2007.00539.x
Wang J, Lu Z-H, Gabius H-J et al (2009) Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol 182:4036–4045. https://doi.org/10.4049/jimmunol.0802981
Wu G, Lu Z-H, André S et al (2016) Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca2+ influx. J Neurochem 136:550–563. https://doi.org/10.1111/jnc.13418
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. https://doi.org/10.1146/annurev.neuro.051508.135600
Kessaris N, Fogarty M, Iannarelli P et al (2006) Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9:173–179. https://doi.org/10.1038/nn1620
Tsai H-H, Niu J, Munji R et al (2016) Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351:379–384. https://doi.org/10.1126/science.aad3839
Goldman SA, Kuypers NJ (2015) How to make an oligodendrocyte. Development 142:3983–3995. https://doi.org/10.1242/dev.126409
Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8:a020453. https://doi.org/10.1101/cshperspect.a020453
Pfeiffer SE, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3:191–197
Zhang S-C, Ge B, Duncan ID (2000) Tracing human oligodendroglial development in vitro. J Neurosci Res 59:421–429. https://doi.org/10.1002/(SICI)1097-4547(20000201)59:3%3c421:AID-JNR17%3e3.0.CO;2-C
Barateiro A, Fernandes A (2014) Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta Mol Cell Res 1843:1917–1929. https://doi.org/10.1016/j.bbamcr.2014.04.018
Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109:61–73
Takebayashi H, Yoshida S, Sugimori M et al (2000) Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev 99:143–148
Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330:779–782. https://doi.org/10.1126/science.1190927
Schauer R, Kamerling JP (2018) Exploration of the sialic acid world. Adv Carbohydr Chem Biochem 75:1–213. https://doi.org/10.1016/BS.ACCB.2018.09.001
Zuber C, Roth J (2009) N-glycosylation. In: Gabius H-J (ed) The sugar code. Fundamentals of glycosciences. Wiley-VCH, Weinheim, pp 87–110
Wang S, Sdrulla AD, diSibio G et al (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21:63–75
Charles P, Hernandez MP, Stankoff B et al (2000) Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci USA 97:7585–7590. https://doi.org/10.1073/pnas.100076197
Baron W, Colognato H, ffrench-Constant C (2005) Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 49:467–479. https://doi.org/10.1002/glia.20132
Miller RH (2002) Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol 67:451–467
Zuchero JB, Barres BA (2013) Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol 23:914–920. https://doi.org/10.1016/j.conb.2013.06.005
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333:1647–1651. https://doi.org/10.1126/science.1206998
Bergles DE, Roberts JDB, Somogyi P, Jahr CE (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405:187–191. https://doi.org/10.1038/35012083
Kukley M, Nishiyama A, Dietrich D (2010) The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci 30:8320–8331. https://doi.org/10.1523/JNEUROSCI.0854-10.2010
Wake H, Ortiz FC, Woo DH et al (2015) Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 6:7844. https://doi.org/10.1038/ncomms8844
Baraban M, Koudelka S, Lyons DA (2018) Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat Neurosci 21:19–23. https://doi.org/10.1038/s41593-017-0040-x
Czopka T, ffrench-Constant C, Lyons DA (2013) Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell 25:599–609. https://doi.org/10.1016/j.devcel.2013.05.013
Simons M, Lyons DA (2013) Axonal selection and myelin sheath generation in the central nervous system. Curr Opin Cell Biol 25:512–519. https://doi.org/10.1016/j.ceb.2013.04.007
Barnett SC, Linington C (2013) Myelination: do astrocytes play a role? Neuroscientist 19:442–450. https://doi.org/10.1177/1073858412465655
Domingues HS, Portugal CC, Socodato R, Relvas JB (2016) Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol 4:71. https://doi.org/10.3389/fcell.2016.00071
Harry GJ, Kraft AD (2012) Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology 33:191–206. https://doi.org/10.1016/j.neuro.2012.01.012
Tanabe S, Yamashita T (2018) B-1a lymphocytes promote oligodendrogenesis during brain development. Nat Neurosci 21:506–516. https://doi.org/10.1038/s41593-018-0106-4
Simpson DL, Thorne DR, Loh HH (1977) Developmentally regulated lectin in neonatal rat brain. Nature 266:367–369
Kobiler D, Beyer EC, Barondes SH (1978) Developmentally regulated lectins from chick muscle, brain, and liver have similar chemical and immunological properties. Dev Biol 64:265–272
Eisenbarth GS, Ruffolo RR, Walsh FS, Nirenberg M (1978) Lactose sensitive lectin of chick retina and spinal cord. Biochem Biophys Res Commun 83:1246–1252
Regan LJ, Dodd J, Barondes SH, Jessell TM (1986) Selective expression of endogenous lactose-binding lectins and lactoseries glycoconjugates in subsets of rat sensory neurons. Proc Natl Acad Sci USA 83:2248–2252. https://doi.org/10.1073/pnas.83.7.2248
Joubert Caron M, Bladier D (1988) Distribution of beta-galactoside specific lectin activities during pre- and post- natal mouse brain development. Cell Mol Biol 34:79–87
Gabius HJ, Wosgien B, Hendrys M, Bardosi A (1991) Lectin localization in human nerve by biochemically defined lectin-binding glycoproteins, neoglycoprotein and lectin-specific antibody. Histochemistry 95:269–277
Jessell TM, Hynes MA, Dodd J (1990) Carbohydrates and carbohydrate-binding proteins in the nervous system. Annu Rev Neurosci 13:227–255. https://doi.org/10.1146/annurev.ne.13.030190.001303
Gaudet AD, Steeves JD, Tetzlaff W, Ramer MS (2005) Expression and functions of galectin-1 in sensory and motoneurons. Curr Drug Targets 6:419–425
Horie H, Inagaki Y, Sohma Y et al (1999) Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci 19:9964–9974. https://doi.org/10.1523/JNEUROSCI.19-22-09964.1999
Mishima T, Hirase H (2010) In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci 30:3093–3100. https://doi.org/10.1523/JNEUROSCI.5065-09.2010
Kopitz J, Russwurm R, Kaltner H et al (2004) Hippocampal neurons and recombinant galectins as tools for systematic carbohydrate structure–function studies in neuronal differentiation. Dev Brain Res 153:189–196. https://doi.org/10.1016/j.devbrainres.2004.08.005
Puche AC, Poirier F, Hair M et al (1996) Role of galectin-1 in the developing mouse olfactory system. Dev Biol 179:274–287. https://doi.org/10.1006/dbio.1996.0257
Dodd J, Jessell TM (1986) Cell surface glycoconjugates and carbohydrate-binding proteins: possible recognition signals in sensory neuron development. J Exp Biol 124:225–238
Pasquini LA, Millet V, Hoyos HC et al (2011) Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ 18:1746–1756. https://doi.org/10.1038/cdd.2011.40
Stancic M, Slijepcevic D, Nomden A et al (2012) Galectin-4, a novel neuronal regulator of myelination. Glia 60:919–935. https://doi.org/10.1002/glia.22324
Velasco S, Díez-Revuelta N, Hernández-Iglesias T et al (2013) Neuronal Galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J Neurochem 125:49–62. https://doi.org/10.1111/jnc.12148
Stechly L, Morelle W, Dessein A-F et al (2009) Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 10:438–450. https://doi.org/10.1111/j.1600-0854.2009.00882.x
Diéz-Revuelta N, Velasco S, André S et al (2010) Phosphorylation of adhesion- and growth-regulatory human galectin-3 leads to the induction of axonal branching by local membrane L1 and ERM redistribution. J Cell Sci 123:671–681. https://doi.org/10.1242/jcs.058198
Mahoney SA, Wilkinson M, Smith S, Haynes LW (2000) Stabilization of neurites in cerebellar granule cells by transglutaminase activity: identification of midkine and galectin-3 as substrates. Neuroscience 101:141–155
Pesheva P, Kuklinski S, Schmitz B, Probstmeier R (1998) Galectin-3 promotes neural cell adhesion and neurite growth. J Neurosci Res 54:639–654. https://doi.org/10.1002/(SICI)1097-4547(19981201)54:5%3c639:AID-JNR9%3e3.0.CO;2-2
White R, Gonsior C, Krämer-Albers E-M et al (2008) Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol 181:579–586. https://doi.org/10.1083/jcb.200706164
Krämer EM, Klein C, Koch T et al (1999) Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem 274:29042–29049
Baron W, Hoekstra D (2010) On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett 584:1760–1770. https://doi.org/10.1016/j.febslet.2009.10.085
Laursen LS, Chan CW, ffrench-Constant C (2009) An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci 29:9174–9185. https://doi.org/10.1523/JNEUROSCI.5942-08.2009
Díez-Revuelta N, Higuero AM, Velasco S et al (2017) Neurons define non-myelinated axon segments by the regulation of galectin-4-containing axon membrane domains. Sci Rep 7:12246. https://doi.org/10.1038/s41598-017-12295-6
Saal I, Nagy N, Lensch M et al (2005) Human galectin-2: expression profiling by RT-PCR/immunohistochemistry and its introduction as a histochemical tool for ligand localization. Histol Histopathol 20:1191–1208. https://doi.org/10.14670/HH-20.1191
Kopitz J, Ballikaya S, André S, Gabius H-J (2012) Ganglioside GM1/galectin-dependent growth regulation in human neuroblastoma cells: special properties of bivalent galectin-4 and significance of linker length for ligand selection. Neurochem Res 37:1267–1276. https://doi.org/10.1007/s11064-011-0693-x
Çolakoğlu G, Bergstrom-Tyrberg U, Berglund EO, Ranscht B (2014) Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci USA 111:E394–E403. https://doi.org/10.1073/pnas.1313769110
Ideo H, Hoshi I, Yamashita K, Sakamoto M (2013) Phosphorylation and externalization of galectin-4 is controlled by Src family kinases. Glycobiology 23:1452–1462. https://doi.org/10.1093/glycob/cwt073
Sperber BR, McMorris FA (2001) Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J Neurosci Res 63:303–312. https://doi.org/10.1002/1097-4547(20010215)63:4%3c303:AID-JNR1024%3e3.0.CO;2-A
Osterhout DJ, Wolven A, Wolf RM et al (1999) Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol 145:1209–1218
Umemori H, Satot S, Yagi T et al (1994) Initial events of myelination involve Fyn tyrosine kinase signalling. Nature 367:572–576. https://doi.org/10.1038/367572a0
Grant SG, Karl KA, Kiebler MA, Kandel ER (1995) Focal adhesion kinase in the brain: novel subcellular localization and specific regulation by Fyn tyrosine kinase in mutant mice. Genes Dev 9:1909–1921
Maier O, Hoekstra D, Baron W (2008) Polarity development in oligodendrocytes: sorting and trafficking of myelin components. J Mol Neurosci 35:35–53. https://doi.org/10.1007/s12031-007-9024-8
Hirahara Y, Bansal R, Honke K et al (2004) Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia 45:269–277. https://doi.org/10.1002/glia.10327
Bansal R, Winkler S, Bheddah S (1999) Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci 19:7913–7924
Baron W, Ozgen H, Klunder B et al (2015) The major myelin-resident protein PLP is transported to myelin membranes via a transcytotic mechanism: involvement of sulfatide. Mol Cell Biol 35:288–302. https://doi.org/10.1128/MCB.00848-14
Ozgen H, Schrimpf W, Hendrix J et al (2014) The lateral membrane organization and dynamics of myelin proteins PLP and MBP are dictated by distinct galactolipids and the extracellular matrix. PLoS One 9:e101834. https://doi.org/10.1371/journal.pone.0101834
Wei Q, Eviatar-Ribak T, Keith Miskimins W, Miskimins R (2007) Galectin-4 is involved in p27-mediated activation of the myelin basic protein promoter. J Neurochem 101:1214–1223. https://doi.org/10.1111/j.1471-4159.2007.04488.x
Wei Q, Miskimins WK, Miskimins R (2003) The Sp1 family of transcription factors is involved in p27(Kip1)-mediated activation of myelin basic protein gene expression. Mol Cell Biol 23:4035–4045
Joubert R, Kuchler S, Zanetta JP et al (1989) Immunohistochemical localization of a β-galactoside-binding lectin in rat central nervous system. Dev Neurosci 11:397–413. https://doi.org/10.1159/000111916
Hoyos HC, Marder M, Ulrich R et al (2016) The Role of Galectin-3: from oligodendroglial differentiation and myelination to demyelination and remyelination processes in a cuprizone-induced demyelination model. Glial Cells Heal Dis CNS. https://doi.org/10.1007/978-3-319-40764-7_15
Ochieng J, Green B, Evans S et al (1998) Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta 1379:97–106
de Vries H, Schrage C, Hoekstra D (1998) An apical-type trafficking pathway is present in cultured oligodendrocytes but the sphingolipid-enriched myelin membrane is the target of a basolateral-type pathway. Mol Biol Cell 9:599–609
Klunder B, Baron W, Schrage C et al (2008) Sorting signals and regulation of cognate basolateral trafficking in myelin biogenesis. J Neurosci Res 86:1007–1016. https://doi.org/10.1002/jnr.21556
Snaidero N, Möbius W, Czopka T et al (2014) Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156:277–290. https://doi.org/10.1016/j.cell.2013.11.044
Rinaldi M, Thomas L, Mathieu P et al (2016) Galectin-1 circumvents lysolecithin-induced demyelination through the modulation of microglial polarization/phagocytosis and oligodendroglial differentiation. Neurobiol Dis 96:127–143. https://doi.org/10.1016/j.nbd.2016.09.003
Kopitz J, von Reitzenstein C, André S et al (2001) Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem 276:35917–35923. https://doi.org/10.1074/jbc.M105135200
Thomas L, Pasquini LA (2018) Extracellular galectin-3 induces accelerated oligodendroglial differentiation through changes in signaling pathways and cytoskeleton dynamics. Mol Neurobiol 56:336–349. https://doi.org/10.1007/s12035-018-1089-6
Salomonsson E, Larumbe A, Tejler J et al (2010) Monovalent interactions of galectin-1. Biochemistry 49:9518–9532. https://doi.org/10.1021/bi1009584
Cho M, Cummings RD (1996) Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry 35:13081–13088. https://doi.org/10.1021/bi961181d
Horie H, Kadoya T, Sango K, Hasegawa M (2005) Oxidized galectin-1 is an essential factor for peripheral nerve regeneration. Curr Drug Targets 6:385–394
Plachta N, Annaheim C, Bissière S et al (2007) Identification of a lectin causing the degeneration of neuronal processes using engineered embryonic stem cells. Nat Neurosci 10:712–719. https://doi.org/10.1038/nn1897
Tracey BM, Feizi T, Abbott WM et al (1992) Subunit molecular mass assignment of 14,654 Da to the soluble β-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J Biol Chem 267:10342–10347
Whitney PL, Powell JT, Sanford GL (1986) Oxidation and chemical modification of lung β-galactoside-specific lectin. Biochem J 238:683–689. https://doi.org/10.1042/bj2380683
Hirabayashi J, Kasai K-I (1991) Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa β-galactoside-binding lectin. J Biol Chem 266:23648–23653
Nishi N, Abe A, Iwaki J et al (2008) Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology 18:1065–1073. https://doi.org/10.1093/glycob/cwn089
Thomas L, Pasquini LA (2018) Galectin-3-mediated glial crosstalk drives oligodendrocyte differentiation and (re)myelination. Front Cell Neurosci 12:297. https://doi.org/10.3389/fncel.2018.00297
Nawaz S, Sánchez P, Schmitt S et al (2015) Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell 34:139–151. https://doi.org/10.1016/j.devcel.2015.05.013
Lalancette-Hebert M, Swarup V, Beaulieu JM et al (2012) Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci 32:10383–10395. https://doi.org/10.1523/JNEUROSCI.1498-12.2012
Zeger M, Popken G, Zhang J et al (2007) Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normalin vivo oligodendrocyte development and myelination. Glia 55:400–411. https://doi.org/10.1002/glia.20469
Galvin J, Eyermann C, Colognato H (2010) Dystroglycan modulates the ability of insulin-like growth factor-1 to promote oligodendrocyte differentiation. J Neurosci Res 88:3295–3307. https://doi.org/10.1002/jnr.22484
McMorris FA, Smith TM, DeSalvo S, Furlanetto RW (1986) Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci USA 83:822–826
Jeffery ND, Blakemore WF (1997) Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain 120:27–37. https://doi.org/10.1093/brain/120.1.27
Zambonin JL, Zhao C, Ohno N et al (2011) Increased mitochondrial content in remyelinated axons: implications for multiple sclerosis. Brain 134:1901–1913. https://doi.org/10.1093/brain/awr110
Smith KJ, Blakemore WF, McDonald WI (1979) Central remyelination restores secure conduction. Nature 280:395–396. https://doi.org/10.1038/280395a0
Zawadzka M, Rivers LE, Fancy SPJ et al (2010) CNS-resident glial progenitor/stem cells produce schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6:578–590. https://doi.org/10.1016/j.stem.2010.04.002
Barbin G, Aigrot MS, Charles P et al (2004) Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol 1:65–72. https://doi.org/10.1017/S1740925X04000092
Claycomb K, Johnson K, Winokur P et al (2013) Astrocyte regulation of CNS inflammation and remyelination. Brain Sci 3:1109–1127. https://doi.org/10.3390/brainsci3031109
de Jong CGHM, Stancic M, Pinxterhuis TH et al (2018) Galectin-4, a negative regulator of oligodendrocyte differentiation, is persistently present in axons and microglia/macrophages in multiple sclerosis lesions. J Neuropathol Exp Neurol 77:1024–1038. https://doi.org/10.1093/jnen/nly081
Hamaguchi M, Muramatsu R, Fujimura H et al (2019) Circulating transforming growth factor-β1 facilitates remyelination in the adult central nervous system. Elife. https://doi.org/10.7554/eLife.41869
Dombrowski Y, O’Hagan T, Dittmer M et al (2017) Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci 20:674–680. https://doi.org/10.1038/nn.4528
Torkildsen Ø, Brunborg LA, Myhr K-M, Bø L (2008) The cuprizone model for demyelination. Acta Neurol Scand 117:72–76. https://doi.org/10.1111/j.1600-0404.2008.01036.x
Praet J, Guglielmetti C, Berneman Z et al (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47:485–505. https://doi.org/10.1016/J.NEUBIOREV.2014.10.004
Jeffery ND, Blakemore WF (1995) Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol 24:775–781. https://doi.org/10.1007/BF01191213
Skripuletz T, Gudi V, Hackstette D, Stangel M (2011) De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 26:1585–1597. https://doi.org/10.14670/HH-26.1585
Blakemore WF (1976) Invasion of Schwann cells into the spinal cord of the rat following local injections of lysolecithin. Neuropathol Appl Neurobiol 2:21–39. https://doi.org/10.1111/j.1365-2990.1976.tb00559.x
Zhao C, Li W-W, Franklin RJM (2006) Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging 27:1298–1307. https://doi.org/10.1016/J.NEUROBIOLAGING.2005.06.008
Muramatsu R, Kuroda M, Matoba K et al (2015) Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system. J Biol Chem 290:11515–11525. https://doi.org/10.1074/jbc.M114.587253
Kondo A, Nakano T, Suzuki K (1987) Blood–brain barrier permeability to horseradish peroxidase in twitcher and cuprizone-intoxicated mice. Brain Res 425:186–190
McMahon EJ, Suzuki K, Matsushima GK (2002) Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood–brain barrier. J Neuroimmunol 130:32–45
Bieber AJ, Kerr S, Rodriguez M (2003) Efficient central nervous system remyelination requires T cells. Ann Neurol 53:680–684. https://doi.org/10.1002/ana.10578
Hiremath MM, Chen VS, Suzuki K et al (2008) MHC class II exacerbates demyelination in vivo independently of T cells. J Neuroimmunol 203:23–32. https://doi.org/10.1016/j.jneuroim.2008.06.034
Skripuletz T, Hackstette D, Bauer K et al (2013) Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136:147–167. https://doi.org/10.1093/brain/aws262
Russo MV, McGavern DB (2015) Immune Surveillance of the CNS following Infection and Injury. Trends Immunol 36:637–650. https://doi.org/10.1016/j.it.2015.08.002
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. https://doi.org/10.12703/p6-13
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm 11:98. https://doi.org/10.1186/1742-2094-11-98
Schwartz M, Butovsky O, Brück W, Hanisch U-K (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29:68–74. https://doi.org/10.1016/J.TINS.2005.12.005
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394. https://doi.org/10.1038/nn1997
Olah M, Amor S, Brouwer N et al (2012) Identification of a microglia phenotype supportive of remyelination. Glia 60:306–321. https://doi.org/10.1002/glia.21266
Kotter MR, Li W-W, Zhao C, Franklin RJM (2006) Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci 26:328–332. https://doi.org/10.1523/JNEUROSCI.2615-05.2006
Robinson S, Miller RH (1999) Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol 216:359–368. https://doi.org/10.1006/dbio.1999.9466
Syed YA, Baer AS, Lubec G et al (2008) Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg Focus 24:E5. https://doi.org/10.3171/FOC/2008/24/3-4/E4
Miron VE, Boyd A, Zhao J-W et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. https://doi.org/10.1038/nn.3469
Lian H, Litvinchuk A, Chiang AC-A et al (2016) Astrocyte-Microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s Disease. J Neurosci 36:577–589. https://doi.org/10.1523/JNEUROSCI.2117-15.2016
Liu W, Tang Y, Feng J (2011) Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci 89:141–146. https://doi.org/10.1016/j.lfs.2011.05.011
Clemente D, Ortega MC, Melero-Jerez C, de Castro F (2013) The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Front Cell Neurosci 7:268. https://doi.org/10.3389/fncel.2013.00268
Kıray H, Lindsay SL, Hosseinzadeh S, Barnett SC (2016) The multifaceted role of astrocytes in regulating myelination. Exp Neurol 283:541–549. https://doi.org/10.1016/J.EXPNEUROL.2016.03.009
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65:2702–2720. https://doi.org/10.1007/s00018-008-8059-5
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Paclik D, Danese S, Berndt U et al (2008) Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS One 3:e2629. https://doi.org/10.1371/journal.pone.0002629
Paclik D, Werner L, Guckelberger O et al (2011) Galectins distinctively regulate central monocyte and macrophage function. Cell Immunol 271:97–103. https://doi.org/10.1016/j.cellimm.2011.06.003
Starossom SC, Mascanfroni ID, Imitola J et al (2012) Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37:249–263. https://doi.org/10.1016/j.immuni.2012.05.023
Hsieh SH, Ying NW, Wu MH et al (2008) Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 27:3746–3753. https://doi.org/10.1038/sj.onc.1211029
Nissen JC, Tsirka SE (2016) Tuftsin-driven experimental autoimmune encephalomyelitis recovery requires neuropilin-1. Glia 64:923–936. https://doi.org/10.1002/glia.22972
Quintá HR, Pasquini JM, Rabinovich GA, Pasquini LA (2014) Glycan-dependent binding of galectin-1 to neuropilin-1 promotes axonal regeneration after spinal cord injury. Cell Death Differ 21:941–955. https://doi.org/10.1038/cdd.2014.14
Syed YA, Hand E, Mobius W et al (2011) Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci 31:3719–3728. https://doi.org/10.1523/JNEUROSCI.4930-10.2011
Sasaki T, Hirabayashi J, Manya H et al (2004) Galectin-1 induces astrocyte differentiation, which leads to production of brain-derived neurotrophic factor. Glycobiology 14:357–363. https://doi.org/10.1093/glycob/cwh043
Qu W, Wang Y, Wang J et al (2010) Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochem Res 35:1716–1724. https://doi.org/10.1007/s11064-010-0234-z
Kajitani K, Nomaru H, Ifuku M et al (2009) Galectin-1 promotes basal and kainate-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ 16:417–427. https://doi.org/10.1038/cdd.2008.162
Hillis JM, Davies J, Mundim MV et al (2016) Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. J Neuroinflamm 13:190. https://doi.org/10.1186/s12974-016-0651-2
Hoyos HC, Rinaldi M, Mendez-Huergo SP et al (2014) Galectin-3 controls the response of microglial cells to limit cuprizone-induced demyelination. Neurobiol Dis 62:441–455. https://doi.org/10.1016/j.nbd.2013.10.023
Hsu DK, Yang R-Y, Pan Z et al (2000) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156:1073–1083. https://doi.org/10.1016/S0002-9440(10)64975-9
Kadrofske MM, Openo KP, Wang JL (1998) The human GALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys 349:7–20. https://doi.org/10.1006/ABBI.1997.0447
Rotshenker S (2009) The role of galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J Mol Neurosci 39:99–103. https://doi.org/10.1007/s12031-009-9186-7
Rotshenker S, Reichert F, Gitik M et al (2008) Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia 56:1607–1613. https://doi.org/10.1002/glia.20713
Jeon S-B, Yoon HJ, Chang CY et al (2010) Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol 185:7037–7046. https://doi.org/10.4049/jimmunol.1000154
Burguillos MA, Svensson M, Schulte T et al (2015) Microglia-secreted galectin-3 acts as a Toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep 10:1626–1638. https://doi.org/10.1016/j.celrep.2015.02.012
Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the central nervous system: structure, function, and pathology. Physiol Rev 99:1381–1431. https://doi.org/10.1152/physrev.00031.2018
Duncan ID, Radcliff AB (2016) Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp Neurol 283:452–475. https://doi.org/10.1016/j.expneurol.2016.04.002
Lassmann H, Brück W, Lucchinetti C (2001) Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 7:115–121. https://doi.org/10.1016/S1471-4914(00)01909-2
Lassmann H, Ransohoff RM (2004) The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol 25:132–137. https://doi.org/10.1016/j.it.2004.01.007
Fletcher JM, Lalor SJ, Sweeney CM et al (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162:1–11. https://doi.org/10.1111/j.1365-2249.2010.04143.x
Lucchinetti C, Brück W, Parisi J et al (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Segal B (2019) The diversity of encephalitogenic CD4+ T cells in multiple sclerosis and its animal models. J Clin Med 8:E120. https://doi.org/10.3390/jcm8010120
Barnett M, Henderson A, Prineas J (2006) The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult Scler J 12:121–132. https://doi.org/10.1191/135248506ms1304rr
Berger C, Hiestand P, Kindler-Baumann D et al (2006) Analysis of lesion development during acute inflammation and remission in a rat model of experimental autoimmune encephalomyelitis by visualization of macrophage infiltration, demyelination and blood–brain barrier damage. NMR Biomed 19:101–107. https://doi.org/10.1002/nbm.1007
Brück W, Sommermeier N, Bergmann M et al (1996) Macrophages in multiple sclerosis. Immunobiology 195:588–600. https://doi.org/10.1016/S0171-2985(96)80024-6
Zrzavy T, Hametner S, Wimmer I et al (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140:1900–1913. https://doi.org/10.1093/brain/awx113
Remington LT, Babcock AA, Zehntner SP, Owens T (2007) Microglial recruitment, activation, and proliferation in response to primary demyelination. Am J Pathol 170:1713–1724. https://doi.org/10.2353/ajpath.2007.060783
Yamasaki R, Lu H, Butovsky O et al (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549. https://doi.org/10.1084/jem.20132477
Zhou X, He X, Ren Y (2014) Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res 9:1787–1795. https://doi.org/10.4103/1673-5374.143423
Greenhalgh AD, Zarruk JG, Healy LM et al (2018) Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol 16:e2005264. https://doi.org/10.1371/journal.pbio.2005264
Trapp BD, Nave K-A (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. https://doi.org/10.1146/annurev.neuro.30.051606.094313
Stadelmann C, Wegner C, Brück W (2011) Inflammation, demyelination, and degeneration—recent insights from MS pathology. Biochim Biophys Acta 1812:275–282. https://doi.org/10.1016/J.BBADIS.2010.07.007
Stys PK, Zamponi GW, van Minnen J, Geurts JJG (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 13:507–514. https://doi.org/10.1038/nrn3275
Brück W (2005) The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol 252:v3–v9. https://doi.org/10.1007/s00415-005-5002-7
Rodriguez M, Scheithauer B (1994) Ultrastructure of multiple sclerosis. Ultrastruct Pathol 18:3–13. https://doi.org/10.3109/01913129409016267
Patrikios P, Stadelmann C, Kutzelnigg A et al (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129:3165–3172. https://doi.org/10.1093/brain/awl217
Luchetti S, Fransen NL, van Eden CG et al (2018) Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 135:511–528. https://doi.org/10.1007/s00401-018-1818-y
Gerritse K, Laman JD, Noelle RJ et al (1996) CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 93:2499–2504
Liu JS, Zhao ML, Brosnan CF, Lee SC (2001) Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol 158:2057–2066. https://doi.org/10.1016/S0002-9440(10)64677-9
Vogel DY, Vereyken EJ, Glim JE et al (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflamm 10:35. https://doi.org/10.1186/1742-2094-10-35
Peferoen LAN, Vogel DYS, Ummenthum K et al (2015) Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol 74:48–63. https://doi.org/10.1097/NEN.0000000000000149
Fawcett JW, Asher R (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391. https://doi.org/10.1016/S0361-9230(99)00072-6
Anderson MA, Burda JE, Ren Y et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. https://doi.org/10.1038/nature17623
Voskuhl RR, Peterson RS, Song B et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522. https://doi.org/10.1523/JNEUROSCI.1514-09.2009
Anderson AC, Anderson DE, Bregoli L et al (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318:1141–1143. https://doi.org/10.1126/science.1148536
Stancic M, van Horssen J, Thijssen VL et al (2011) Increased expression of distinct galectins in multiple sclerosis lesions. Neuropathol Appl Neurobiol 37:654–671. https://doi.org/10.1111/j.1365-2990.2011.01184.x
Masuda T, Sankowski R, Staszewski O et al (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566:388–392. https://doi.org/10.1038/s41586-019-0924-x
Liu F-T (2005) Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol 136:385–400. https://doi.org/10.1159/000084545
Kang Z, Wang C, Zepp J et al (2013) Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci 16:1401–1408. https://doi.org/10.1038/nn.3505
Baker D, Amor S (2014) Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult Scler Relat Disord 3:555–564. https://doi.org/10.1016/j.msard.2014.05.002
Nagelkerken L (1998) Role of Th1 and Th2 cells in autoimmune demyelinating disease. Brazilian J Med Biol Res 31:55–60. https://doi.org/10.1590/s0100-879x1998000100007
Lafaille JJ, Keere FV, Hsu AL et al (1997) Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med 186:307–312
Kohm AP, Carpentier PA, Miller SD (2003) Regulation of experimental autoimmune encephalomyelitis (EAE) by CD4+CD25+ regulatory T cells. Novartis Found Symp 252:45–52; discussion 52–54, 106–114
Koutrolos M, Berer K, Kawakami N et al (2014) Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun 2:163. https://doi.org/10.1186/S40478-014-0163-1
Baranzini SE, Jeong MC, Butunoi C et al (1999) B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 163:5133–5144
Offner H, Celnik B, Bringman TS et al (1990) Recombinant human β-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol 28:177–184. https://doi.org/10.1016/0165-5728(90)90032-I
Toscano MA, Bianco GA, Ilarregui JM et al (2007) Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol 8:825–834. https://doi.org/10.1038/ni1482
Mari ER, Rasouli J, Ciric B et al (2016) Galectin-1 is essential for the induction of MOG35–55-based intravenous tolerance in experimental autoimmune encephalomyelitis. Eur J Immunol 46:1783–1796. https://doi.org/10.1002/eji.201546212
Lepelletier Y, Lecourt S, Renand A et al (2010) Galectin-1 and semaphorin-3A are two soluble factors conferring T-cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev 19:1075–1079. https://doi.org/10.1089/scd.2009.0212
Jiang H-R, Al Rasebi Z, Mensah-Brown E et al (2009) Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol 182:1167–1173
Demetriou M, Granovsky M, Quaggin S, Dennis JW (2001) Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409:733–739. https://doi.org/10.1038/35055582
Lee S-U, Grigorian A, Pawling J et al (2007) N-Glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem 282:33725–33734. https://doi.org/10.1074/jbc.M704839200
Stillman BN, Hsu DK, Pang M et al (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176:778–789. https://doi.org/10.4049/JIMMUNOL.176.2.778
Reichert F, Rotshenker S (1999) Galectin-3/MAC-2 in experimental allergic encephalomyelitis. Exp Neurol 160:508–514. https://doi.org/10.1006/exnr.1999.7229
Dong S, Hughes RC (1997) Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen). Glycoconj J 14:267–274
Sano H, Hsu DK, Apgar JR et al (2003) Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112:389–397. https://doi.org/10.1172/JCI17592
MacKinnon AC, Farnworth SL, Hodkinson PS et al (2008) Regulation of alternative macrophage activation by galectin-3. J Immunol 180:2650–2658
Novak R, Dabelic S, Dumic J (2012) Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim Biophys Acta 1820:1383–1390. https://doi.org/10.1016/j.bbagen.2011.11.014
Prins CA, Almeida FM, Martinez AMB (2016) Absence of galectin-3 attenuates neuroinflammation improving functional recovery after spinal cord injury. Neural Regen Res 11:92–93. https://doi.org/10.4103/1673-5374.175051
Zhu C, Anderson AC, Schubart A et al (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6:1245–1252. https://doi.org/10.1038/ni1271
Monney L, Sabatos CA, Gaglia JL et al (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541. https://doi.org/10.1038/415536a
Rangachari M, Zhu C, Sakuishi K et al (2012) Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat Med 18:1394–1400. https://doi.org/10.1038/nm.2871
Koguchi K, Anderson DE, Yang L et al (2006) Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med 203:1413–1418. https://doi.org/10.1084/jem.20060210
Saresella M, Piancone F, Marventano I et al (2014) A role for the TIM-3/GAL-9/BAT3 pathway in determining the clinical phenotype of multiple sclerosis. FASEB J 28:5000–5009. https://doi.org/10.1096/fj.14-258194
Hamana A, Takahashi Y, Tanioka A et al (2018) Safe and effective interferon-beta gene therapy for the treatment of multiple sclerosis by regulating biological activity through the design of interferon-beta-galectin-9 fusion proteins. Int J Pharm 536:310–317. https://doi.org/10.1016/j.ijpharm.2017.12.010
Pardo E, Cárcamo C, Uribe-San Martín R et al (2017) Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS One 12:e0177472. https://doi.org/10.1371/journal.pone.0177472
Lutomski D, Joubert-Caron R, Lefebure C et al (1997) Anti-galectin-1 autoantibodies in serum of patients with neurological diseases. Clin Chim Acta 262:131–138
Sarter K, Janko C, André S et al (2013) Autoantibodies against galectins are associated with antiphospholipid syndrome in patients with systemic lupus erythematosus. Glycobiology 23:12–22. https://doi.org/10.1093/glycob/cws120
Mycko MP, Sliwinska B, Cichalewska M et al (2014) Brain glycolipids suppress T helper cells and inhibit autoimmune demyelination. J Neurosci 34:8646–8658. https://doi.org/10.1523/JNEUROSCI.0885-14.2014
Kuhlmann T, Ludwin S, Prat A et al (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133:13–24. https://doi.org/10.1007/s00401-016-1653-y
Raine CS, Wu E (1993) Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol 52:199–204
Ponath G, Park C, Pitt D (2018) The role of astrocytes in multiple sclerosis. Front Immunol 9:217. https://doi.org/10.3389/fimmu.2018.00217
Rinaldi M, Thomas L, Pasquini LA (2016) Galectin-1 in myelin repair. Oncotarget 7:81979–81980. https://doi.org/10.18632/oncotarget.13455
Peterson JW, Bö L, Mörk S et al (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Bø L, Vedeler CA, Nyland H et al (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler J 9:323–331. https://doi.org/10.1191/1352458503ms917oa
Neumann H, Medana IM, Bauer J, Lassmann H (2002) Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci 25:313–319
Steelman AJ, Li J (2014) Astrocyte galectin-9 potentiates microglial TNF secretion. J Neuroinflamm 11:144. https://doi.org/10.1186/s12974-014-0144-0
Steelman AJ, Smith R, Welsh CJ et al (2013) Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J Biol Chem 288:23776–23787. https://doi.org/10.1074/jbc.M113.451658
Yoshida H, Imaizumi T, Kumagai M et al (2001) Interleukin-1β stimulates galectin-9 expression in human astrocytes. NeuroReport 12:3755–3758
Burman J, Svenningsson A (2016) Cerebrospinal fluid concentration of Galectin-9 is increased in secondary progressive multiple sclerosis. J Neuroimmunol 292:40–44. https://doi.org/10.1016/j.jneuroim.2016.01.008
Deshmukh VA, Tardif V, Lyssiotis CA et al (2013) A regenerative approach to the treatment of multiple sclerosis. Nature 502:327–332. https://doi.org/10.1038/nature12647
Plemel JR, Liu W-Q, Yong VW (2017) Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 16:617–634. https://doi.org/10.1038/nrd.2017.115
Amano M, Eriksson H, Manning JC et al (2012) Tumour suppressor p16INK4a—anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J 279:4062–4080. https://doi.org/10.1111/febs.12001
Boscher C, Dennis JW, Nabi IR (2011) Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol 23:383–392. https://doi.org/10.1016/j.ceb.2011.05.001
Ferrer CM, Reginato MJ (2014) Sticking to sugars at the metastatic site: sialyltransferase ST6GalNAc2 acts as a breast cancer metastasis suppressor. Cancer Discov 4:275–277. https://doi.org/10.1158/2159-8290.CD-14-0075
Valenzuela HF, Pace KE, Cabrera PV et al (2007) O-Glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res 67:6155–6162. https://doi.org/10.1158/0008-5472.CAN-05-4431
Petrosyan A, Holzapfel MS, Muirhead DE, Cheng P-W (2014) Restoration of compact golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin O-glycan synthesis. Mol Cancer Res 12:1704–1716. https://doi.org/10.1158/1541-7786.MCR-14-0291-T
Tzeng S-F, Tsai C-H, Chao T-K et al (2018) O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J 32:6869–6882. https://doi.org/10.1096/fj.201800687
Mkhikian H, Grigorian A, Li CF et al (2011) Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun 2:334. https://doi.org/10.1038/ncomms1333
Perillo NL, Pace KE, Seilhamer JJ, Baum LG (1995) Apoptosis of T cells mediated by galectin-1. Nature 378:736–739. https://doi.org/10.1038/378736a0
Pace KE, Hahn HP, Pang M et al (2000) CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J Immunol 165:2331–2334
Ludwig A-K, Kaltner H, Kopitz J, Gabius H-J (2019) Lectinology 4.0: altering modular (ga)lectin display for functional analysis and biomedical applications. Biochim Biophys Acta 1863:935–940. https://doi.org/10.1016/j.bbagen.2019.03.005
Elkon K, Casali P (2008) Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4:491–498. https://doi.org/10.1038/ncprheum0895
Naparstek Y, Plotz PH (1993) The role of autoantibodies in autoimmune disease. Annu Rev Immunol 11:79–104. https://doi.org/10.1146/annurev.iy.11.040193.000455
Nishihara H, Shimizu F, Kitagawa T et al (2017) Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult Scler J 23:382–394. https://doi.org/10.1177/1352458516655217
Yıldırım C, Vogel DYS, Hollander MR et al (2015) Galectin-2 induces a proinflammatory, anti-arteriogenic phenotype in monocytes and macrophages. PLoS One 10:e0124347. https://doi.org/10.1371/journal.pone.0124347
Sturm A, Lensch M, Andre S et al (2004) Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol 173:3825–3837. https://doi.org/10.4049/jimmunol.173.6.3825
Schnaar RL, Lopez PHH (2009) Myelin-associated glycoprotein and its axonal receptors. J Neurosci Res 87:3267–3276. https://doi.org/10.1002/jnr.21992
Kaltner H, Abad-Rodriguez J, Corfield AP et al (2019) The sugar code: letters and vocabulary, writers, editors and readers and biosignificance of functional glycan-lectin pairing. Biochem J 475:2623–2655. https://doi.org/10.1042/BCJ20170853
Sirko S, Irmler M, Gascón S et al (2015) Astrocyte reactivity after brain injury: the role of galectins 1 and 3. Glia 63:2340–2361. https://doi.org/10.1002/glia.22898
Doverhag C, Hedtjärn M, Poirier F et al (2010) Galectin-3 contributes to neonatal hypoxic–ischemic brain injury. Neurobiol Dis 38:36–46. https://doi.org/10.1016/J.NBD.2009.12.024
Satoh K, Niwa M, Goda W et al (2011) Galectin-3 expression in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia, and its inhibition by hypothermia. Brain Res 1382:266–274. https://doi.org/10.1016/j.brainres.2011.01.049
Acknowledgements
Work in the Baron Laboratory is supported by Grants from the Dutch MS Research Foundation (Stichting MS Research).
Author information
Authors and Affiliations
Contributions
CGHMdJ and WB had the idea for the article; CGHMdJ, HJG and WB performed the literature search and data analysis; CGHMdJ drafted the work; HJG and WB critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Jong, C.G.H.M., Gabius, HJ. & Baron, W. The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell. Mol. Life Sci. 77, 1289–1317 (2020). https://doi.org/10.1007/s00018-019-03327-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03327-7