Abstract
Mps One binder 1 (MOB1) is a core component of NDR/LATS kinase and a positive regulator of the Hippo signaling pathway. However, its role in neurite outgrowth still remains to be clarified. Here, we confirmed, for the first time, that MOB1 promoted neurite outgrowth and was involved in functional recovery after spinal cord injury (SCI) in mice. Mechanistically, we found that MOB1 stability was regulated by the PTEN–GSK3β axis. The MOB1 protein was significantly up-regulated in PTEN-knockdown neuronal cells. This effect was dependent on the lipid phosphatase activity of PTEN. Moreover, MOB1 was found to be a novel substrate for GSK3β that is phosphorylated on serine 146 and degraded via the ubiquitin–proteasome system (UPS). Finally, in vivo lentiviral-mediated silencing of PTEN promoted neurite outgrowth and functional recovery after SCI and this effect was reversed by down-regulation of MOB1. Taken together, this study provided mechanistic insight into how MOB1 acts as a novel and a necessary regulator in PTEN–GSK3β axis that controls neurite outgrowth after SCI.








Similar content being viewed by others
References
Gwak SJ, Macks C, Jeong DU, Kindy M, Lynn M, Webb K, Lee JS (2017) RhoA knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model. Biomaterials 121:155–166. https://doi.org/10.1016/j.biomaterials.2017.01.003
McDonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet 359(9304):417–425. https://doi.org/10.1016/s0140-6736(02)07603-1
Lu Y, Belin S, He Z (2014) Signaling regulations of neuronal regenerative ability. Curr Opin Neurobiol 27:135–142. https://doi.org/10.1016/j.conb.2014.03.007
Egawa N, Lok J, Washida K, Arai K (2017) Mechanisms of axonal damage and repair after central nervous system injury. Transl Stroke Res 8(1):14–21. https://doi.org/10.1007/s12975-016-0495-1
Luca FC, Winey M (1998) MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell 9(1):29–46
Hergovich A, Kohler RS, Schmitz D, Vichalkovski A, Cornils H, Hemmings BA (2009) The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr Biol 19(20):1692
Wilmeth LJ, Shrestha S, Montaño G, Rashe J, Shuster CB (2010) Mutual dependence of Mob1 and the chromosomal passenger complex for localization during mitosis. Mol Biol Cell 21(3):380
Florindo C, Perdigão J, Fesquet D, Schiebel E, Pines J, Tavares ÁA (2012) Human Mob1 proteins are required for cytokinesis by controlling microtubule stability. J Cell Sci 125(13):3085–3090. https://doi.org/10.1242/jcs.097147
Hergovich A (2016) The roles of NDR protein kinases in Hippo signalling. Genes 7(5):21
Hergovich A (2011) MOB control: reviewing a conserved family of kinase regulators. Cell Signal 23(9):1433–1440. https://doi.org/10.1016/j.cellsig.2011.04.007
Yang R, Kong E, Jin J, Hergovich A, Püschel AW (2014) Rassf5 and Ndr kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway. J Cell Sci 127(Pt 16):3463
Stork O, Zhdanov A, Kudersky A, Yoshikawa T, Obata K, Pape HC (2004) Neuronal functions of the novel serine/threonine kinase Ndr2. J Biol Chem 279(44):45773
Lin CH, Hsieh M, Fan SS (2011) The promotion of neurite formation in Neuro2A cells by mouse Mob2 protein. FEBS Lett 585(3):523–530
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13(9):1075–1081. https://doi.org/10.1038/nn.2603
Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A, Xu N, Creger D, Liu K, He Z, Zheng B (2015) Effects of PTEN and Nogo codeletion on corticospinal axon sprouting and regeneration in mice. J Neurosci 35(16):6413–6428. https://doi.org/10.1523/JNEUROSCI.4013-14.2015
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296(5573):1655–1657. https://doi.org/10.1126/science.296.5573.1655
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7(4):261
Miao L, Yang L, Huang H, Liang F, Ling C, Hu Y (2016) mTORC1 is necessary but mTORC2 and GSK3beta are inhibitory for AKT3-induced axon regeneration in the central nervous system. eLife 5:e14908. https://doi.org/10.7554/elife.14908
Zhao T, Qi Y, Li Y, Xu K (2012) PI3 Kinase regulation of neural regeneration and muscle hypertrophy after spinal cord injury. Mol Biol Rep 39(4):3541–3547. https://doi.org/10.1007/s11033-011-1127-1
Kim YT, Hur EM, Snider WD, Zhou FQ (2011) Role of GSK3 signaling in neuronal morphogenesis. Front Mol Neurosci 4:48. https://doi.org/10.3389/fnmol.2011.00048
Zhou FQ (2005) CELL BIOLOGY: GSK-3 and microtubule assembly in axons. Science 308(5719):211–214. https://doi.org/10.1126/science.1110301
Byun J, Kim BT, Kim YT, Jiao Z, Hur EM, Zhou FQ (2012) Slit2 inactivates GSK3beta to signal neurite outgrowth inhibition. PLoS One 7(12):e51895. https://doi.org/10.1371/journal.pone.0051895
Liz MA, Mar FM, Santos TE, Pimentel HI, Marques AM, Morgado MM, Vieira S, Sousa VF, Pemble H, Wittmann T, Sutherland C, Woodgett JR, Sousa MM (2014) Neuronal deletion of GSK3beta increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2. BMC Biol 12:47. https://doi.org/10.1186/1741-7007-12-47
Saijilafu Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ (2013) PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun 4(10):2690
Li CL, Sathyamurthy A, Oldenborg A, Tank D, Ramanan N (2014) SRF phosphorylation by glycogen synthase kinase-3 promotes axon growth in hippocampal neurons. J Neurosci 34(11):4027–4042. https://doi.org/10.1523/JNEUROSCI.4677-12.2014
Gobrecht P, Leibinger M, Andreadaki A, Fischer D (2014) Sustained GSK3 activity markedly facilitates nerve regeneration. Nat Commun 5:4561
Di CA, Pesce B, Cordon-Cardo C, Pandolfi PP (1998) Pten is essential for embryonic development and tumour suppression. Nat Genet 19(4):348
Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y (2005) Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120(5):675–685. https://doi.org/10.1016/j.cell.2004.12.036
Ni L, Zheng Y, Hara M, Pan D, Luo X (2015) Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev 29(13):1416–1431. https://doi.org/10.1101/gad.264929.115
Musatov S, Roberts J, Brooks AI, Pena J, Betchen S, Pfaff DW, Kaplitt MG (2004) Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc Natl Acad Sci USA 101(10):3627–3631. https://doi.org/10.1073/pnas.0308289101
Munderloh C, Solis GP, Bodrikov V, Jaeger FA, Wiechers M, Malaga-Trillo E, Stuermer CA (2009) Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J Neurosci 29(20):6607–6615. https://doi.org/10.1523/JNEUROSCI.0870-09.2009
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20(2):84–91
Lin W, Li M, Li Y, Sun X, Li X, Yang F, Huang Y, Wang X (2014) Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci 35(3):449–457. https://doi.org/10.1007/s10072-013-1490-x
Okumura K, Cavenee WK (2005) Cellular transformation by the MSP58 oncogene is inhibited by its physical interaction with the PTEN tumor suppressor. Proc Natl Acad Offences USA 102(8):2703
Tibarewal P, Zilidis G, Spinelli L, Schurch N, Maccario H, Gray A, Perera NM, Davidson L, Barton GJ, Leslie NR (2012) PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal 5(213):ra18
Myers MP, Pass I, Batty IH, Kaay JVD, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK (1998) The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA 95(23):13513–13518
Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL (1998) The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 95(26):15587
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269(7):5241–5248
Dangelmaier C, Manne BK, Liverani E, Jin J, Bray P, Kunapuli SP (2014) PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb Haemost 111(3):508–517
Gills JJ, Dennis PA (2009) Perifosine: Update on a novel Akt inhibitor. Curr Oncol Rep 11(2):102–110
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P (1996) Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272(5264):1023–1026
Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung M, PiwnicaWorms H (2008) GSK-3β targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3β inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell 13(1):36–47
Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A, Lin K, Zhang S, Zhang N, Gottardi CJ, Huang S (2016) Wnt-induced deubiquitination FoxM1 ensures nucleus beta-catenin transactivation. EMBO J 35(6):668–684. https://doi.org/10.15252/embj.201592810
Lignitto L, Arcella A, Sepe M, Rinaldi L, Delle Donne R, Gallo A, Stefan E, Bachmann VA, Oliva MA, Tiziana Storlazzi C, L’Abbate A, Brunetti A, Gargiulo S, Gramanzini M, Insabato L, Garbi C, Gottesman ME, Feliciello A (2013) Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun 4:1822. https://doi.org/10.1038/ncomms2791
Frame S, Cohen P (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359:1–16. https://doi.org/10.1042/0264-6021:3590001
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116(7):1175–1186
Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN (2012) Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in controlling dendrite arborization and spine development. Neuron 73(6):1127–1142
Ohtake Y, Hayat U, Li S (2015) PTEN inhibition and axon regeneration and neural repair. Neural Regen Res 10(9):1363–1368. https://doi.org/10.4103/1673-5374.165496
Huang Z, Hu Z, Xie P, Liu Q (2017) Tyrosine-mutated AAV2-mediated shRNA silencing of PTEN promotes axon regeneration of adult optic nerve. PLoS One 12(3):e0174096. https://doi.org/10.1371/journal.pone.0174096
Wyatt LA, Filbin MT, Keirstead HS (2014) PTEN inhibition enhances neurite outgrowth in human embryonic stem cell-derived neuronal progenitor cells. J Comp Neurol 522(12):2741
Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim CM, Choi YW, Lee YC (2015) Emodin induces neurite outgrowth through PI3K/Akt/GSK-3beta-mediated signaling pathways in Neuro2a cells. Neurosci Lett 588:101–107. https://doi.org/10.1016/j.neulet.2015.01.001
Ding Y, Song Z, Liu J (2016) Aberrant LncRNA expression profile in a contusion spinal cord injury mouse model. Biomed Res Int 2016:9249401. https://doi.org/10.1155/2016/9249401
Chen CH, Sung CS, Huang SY, Feng CW, Hung HC, Yang SN, Chen NF, Tai MH, Wen ZH, Chen WF (2016) The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp Neurol 278:27–41
Danilov CA, Steward O (2015) Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol 266:147–160. https://doi.org/10.1016/j.expneurol.2015.02.012
Park KK, Liu K, Hu Y, Kanter JL, He Z (2010) PTEN/mTOR and axon regeneration. Exp Neurol 223(1):45–50. https://doi.org/10.1016/j.expneurol.2009.12.032
Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, Xu XM (2012) Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One 7(1):e30012
Liu NK, Xu XM (2012) Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regen Res 7(26):2051–2062. https://doi.org/10.3969/j.issn.1673-5374.2012.26.007
Zhang QG, Wu DN, Han D, Zhang GY (2007) Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2 signaling in ischemic brain injury. FEBS Lett 581(3):495–505
Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, Mckay RM, Parada LF (2012) Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci 32(17):5880
Otsubo K, Goto H, Nishio M, Kawamura K, Yanagi S, Nishie W, Sasaki T, Maehama T, Nishina H, Mimori K, Nakano T, Shimizu H, Mak TW, Nakao K, Nakanishi Y, Suzuki A (2017) MOB1-YAP1/TAZ-NKX2.1 axis controls bronchioalveolar cell differentiation, adhesion and tumour formation. Oncogene. https://doi.org/10.1038/onc.2017.58
Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, Guo H, van Gorp PR, Wang DZ, Pu WT (2015) Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res 116(1):35
Fan R, Kim NG, Gumbiner BM (2013) Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA 110(7):2569–2574. https://doi.org/10.1073/pnas.1216462110
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11(8):539–551. https://doi.org/10.1038/nrn2870
Wagner U, Utton M, Gallo JM, Miller CC (1996) Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 109(Pt 6):1537–1543
Barnat M, Benassy MN, Vincensini L, Soares S, Fassier C, Propst F, Andrieux A, Von BY, Nothias F (2016) The GSK3-MAP1B pathway controls neurite branching and microtubule dynamics. Mol Cell Neurosci 72:9–21
Hunter T (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28(5):730
Duan S, Yuan G, Liu X, Ren R, Li J, Zhang W, Wu J, Xu X, Fu L, Li Y, Yang J, Zhang W, Bai R, Yi F, Suzuki K, Gao H, Esteban CR, Zhang C, Izpisua Belmonte JC, Chen Z, Wang X, Jiang T, Qu J, Tang F, Liu GH (2015) PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype. Nat Commun 6:10068. https://doi.org/10.1038/ncomms10068
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Acknowledgements
We thank Dawei Wang for his help in some experiments. We also thank Nida Fatima Moazzam (Ph.D.) for her language guidance during the revision of this manuscript. This study was supported by grants from the National Science Foundation of China (81471263), the Natural Science Foundation of Jiangsu Province (BK20151177), Changzhou High-Level Medical Talents Training Project (2016ZCLJ005), the Postdoctoral science foundation of China (2016M600383), and the Special Funded Projects of China Postdoctoral Fund (2017T100337).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
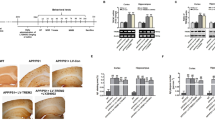
Figure S1. PTEN-GSK3β-MOB1 axis regulates neurite outgrowth in mouse primary hippocampal neurons.
(a) Immunofluorescence staining (anti-NF200) and morphological photographs of primary hippocampal neurons. Scale bar, 250 μm. (b, c) Representative immunofluorescence with anti-NF200 for hippocampal neurons infected with Lenti-sh-PTEN or Lenti-control, respectively. Scale bar, 100 μm (b). The quantification of the neurite length in hippocampal neurons stained with NF200-Cy3 (*P < 0.05, T-test) (c). (d, e) Representative immunofluorescence with anti-NF200 in hippocampal neurons infected with Lenti-MOB1A or Lenti-control, respectively. Scale bar, 100 μm (d). The quantification of the neurite length for hippocampal neurons stained with NF200-Cy3 (*P < 0.05, T-test) (e). (f, g) PTEN, MOB1, NF200, p-GSK3β, GSK3β, GAP43 and p-GAP43 protein levels were checked from the hippocampal neurons which were infected with Lenti-sh-PTEN or Lenti-control by using western blotting. GAPDH used as an internal control (*P < 0.05, T-test). (h, i) NF200, MOB1, GAP43 and p-GAP43 protein levels were checked from the hippocampal neurons which were infected with Lenti-MOB1A or Lenti-control by using western blotting. GAPDH used as an internal control (*P < 0.05, T-test). (j) endogenous MOB1 interacts with GSK3β in hippocampal neurons. Cell extracts of neurons were subjected to IP by the MOB1 antibody or control IgG, followed by western blotting with anti-GSK3β antibody. (k) Double immunofluorescence staining with anti-GSK3β and anti-MOB1A in hippocampal neurons. Scale bar, 50 μm (TIFF 1736 kb)
Figure S2. PTEN-regulated MOB1 expression has an effect on the neurite outgrowth and formation of glial scar following SCI.
(a, b) H&E images of the spinal cord longitudinal sections showing the lesion size in the various groups (1 week after SCI). Scale bar, 1mm (a). Quantitative analysis of lesion size in three SCI groups (P >0.05 versus Lenti-eGFP group, ANOVA test) (b). (c, d) Western blot analysis showing relative expression of PTEN and MOB1 after SCI in the various groups (1 week after SCI), and GAPDH served as an internal control (*P < 0.05 vs Lenti-eGFP group, #P < 0.05 vs Lenti-sh-PTEN group, ANOVA test followed by Dunnett’s Post-Hoc test). (e, f) Fluorescent images of the longitudinal sections of spinal cord showing GFAP-Cy3 labeled astrocytes in the various groups at 4 weeks after SCI) (upper panels, lower magnifications. scale bar, 1mm) (low panels, higher magnifications, scale bar, 250μm) (e). Quantitative analysis of the GFAP+ cells in the central part of longitudinal sections of the spinal cord (*P < 0.05 vs Lenti-sh-PTEN group, ANOVA test followed by Dunnett’s Post-Hoc test). (f). (g) The expression of MOB1 in different nerve cells at 4 weeks after SCI by double immunofluorescence staining (MOB1-Cy3, NeuN-FITC, GFAP-FITC, Olig2-FITC) (upper panels, lower magnifications. scale bar, 1mm) (low panels, higher magnifications, arrows indicated positive cells. scale bar, 250 μm) (TIFF 2724 kb)
Rights and permissions
About this article
Cite this article
Song, Z., Han, X., Zou, H. et al. PTEN–GSK3β–MOB1 axis controls neurite outgrowth in vitro and in vivo. Cell. Mol. Life Sci. 75, 4445–4464 (2018). https://doi.org/10.1007/s00018-018-2890-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2890-0