Abstract
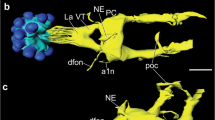
We report identification of a beta-type pigment-dispersing hormone (PDH) identical in two water flea species, Daphnia magna and Daphnia pulex. It has been identified by cloning of precursors, chromatographic isolation from tissue extracts followed by immunoassays and de novo-mass spectrometric sequencing. The peptide is restricted to a complex system of distinct interneurons in the brain and visual ganglia, but does not occur in neurosecretory cells projecting to neurohemal organs as in decapod crustaceans. Thirteen neuron types individually identified and reconstructed by immunohistochemistry were almost identical in terms of positions and projection patterns in both species. Several neurons invade and form plexuses in visual ganglia and major brain neuropils including the central body. Five neuron types show contralateral pathways and form plexuses in the lateral, dorsal, or postlateral brain neuropils. Others are local interneurons, and a tritocerebral neuron connects the protocerebrum with the neuropil of the locomotory second antenna. Two visual ganglia neuron types lateral to the medulla closely resemble insect medulla lateral circadian clock neurons containing pigment-dispersing factor based upon positional and projectional criteria. Experiments under 12:12 h light/dark cycles and constant light or darkness conditions showed significant circadian changes in numbers and activities of one type of medulla lateral PDH neuron with an acrophase in the evening. This simple PDH system shows striking homologies to PDH systems in decapod crustaceans and well-known clock neurons in several insects, which suggests evolutionary conservation of an ancient peptidergic interneuronal system that is part of biological clocks.










Similar content being viewed by others
Abbreviations
- a2n1 :
-
Second antenna nerve 1
- BrOG:
-
Brain-optic ganglia complex
- CCAP:
-
Crustacean cardioactive peptide
- CEC:
-
Circumesophageal commissure
- CHH:
-
Crustacean hyperglycemic hormone
- CID:
-
Collision-induced dissociation
- CB:
-
Central body
- CN:
-
Central neuropil
- CNS:
-
Central nervous system
- CT:
-
Circadian time
- DAPI:
-
4′,6′ Diamino-2-phenylindole-2 HCl
- DD:
-
Constant darkness condition
- DN:
-
Dorsal neuropil
- DVM:
-
Diel vertical migration
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPR:
-
Extra-retinal photoreceptor
- EST:
-
Expressed sequence tag
- HPLC:
-
High-pressure lipid chromatography
- IEP:
-
Isoelectric point
- IIF:
-
Indirect immunofluorescence
- La:
-
Lamina
- LD:
-
Light dark conditions
- LL:
-
Constant light condition
- l-LNvs:
-
Large ventral lateral neurons
- LN:
-
Lateral neuropil
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionization-time of flight
- Me:
-
Medulla
- Mel:
-
Medulla lateral neurons
- MLL:
-
Levator labri muscle
- NE:
-
Nauplius eye
- ORF:
-
Open-reading frame
- PAP:
-
Peroxidase anti-peroxidase
- PB:
-
Protocerebral bridge
- PCN:
-
Precentral neuropil
- PDF:
-
Pigment-dispersing factor
- PDH:
-
Pigment-dispersing hormone
- pec:
-
Post-esophageal commissure
- PER:
-
Period gene product
- PLN:
-
Postlateral neuropils
- PPRP:
-
PDH-precursor-related peptide
- RACE:
-
Rapid amplification of cDNA ends
- RPCH:
-
Red pigment-concentrating hormone
- RT:
-
Room temperature
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- s-LNvs:
-
Small lateral ventral neurons
- SP:
-
Signal peptide
- VNC:
-
Ventral nerve cord
- ZT:
-
Zeitgeber time
References
Rao KR, Riehm JP (1993) Pigment-dispersing hormones. Ann NY Acad Sci 680:78–88
Rao KR (2001) Crustacean pigmentary-effector hormones: chemistry and functions of RPCH, PDH, and related peptides. Am Zool 41(3):364–379
Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnson L, Tarr GE (1987) Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. J Biol Chem 262(6):2672–2675
Rao KR, Riehm JP (1989) The pigment-dispersing hormone family: chemistry, structure-activity relations, and distribution. Biol Bull 177:225–229
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against synthetic PDH. Cell Tissue Res 250:377–387
Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP (1987) Immunocytochemical localization of pigment-dispersing hormone (PDH) and its coexistence with FMRFamide-immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res 250:365–375
Hsu Y-WA, Stemmler EA, Messinger DI, Dickinson PS, Christie AE, de la Iglesia HO (2008) Cloning and differential expression of two beta-pigment-dispersing hormone (beta-PDH) isoforms in the crab Cancer productus: evidence for authentic beta-PDH as a local neurotransmitter and beta-PDH II as a humoral factor. J Comp Neurol 508(2):197–211
Mangerich S, Keller R (1988) Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea), with reference to FMRFamide immunoreactivity in O. limosus. Cell Tissue Res 253(1):199–208
Harzsch S, Dircksen H, Beltz BS (2009) Development of pigment-dispersing hormone-immunoreactive neurons in the American lobster: homology to the insect circadian pacemaker system? Cell Tissue Res 335(2):417–429
Nussbaum T, Dircksen H (1995) Neuronal pathways of classical crustacean neurohormones in the central nervous system of the woodlouse, Oniscus asellus (L). Phil Trans R Soc Lond B 347:139–154
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92(2):612–616
Helfrich-Förster C (2005) Organization of endogenous clocks in insects. Biochem Soc Trans 33(5):957–961
Homberg U, Reischig T, Stengl M (2003) Neural organization of the circadian system of the cockroach Leucophaea maderae. Chronobiol Int 20(4):577–591
Tomioka K, Matsumoto A (2010) A comparative view of insect circadian clock systems. Cell Mol Life Sci 67(9):1397–1406. doi:10.1007/s00018-009-0232-y
Helfrich-Förster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15(6):567–594
Meinertzhagen IA, Pyza E (1996) Daily rhythms in cells of the fly’s optic lobe: taking time out from the circadian clock. Trends Neurosci 19(7):285–291
Strauß J, Dircksen H (2010) Circadian clocks in crustaceans: identified neuronal and cellular systems. Front Biosci 15:1040–1074
Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci USA 97(7):3608–3613. doi:10.1073/pnas.070036197
Zavodska R, Wen C-J, Hrdy I, Sauman I, Lee H-J, Sehnal F (2008) Distribution of corazonin and pigment-dispersing factor in the cephalic ganglia of termites. Arthropod Struct Dev 37:273–286
Sullivan JM, Genco MC, Marlow ED, Benton JL, Beltz BS, Sandeman DC (2009) Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol Int 26(6):1136–1168. doi:10.1080/07420520903217960
Ståhl F (1938) Preliminary report on the colour changes and the incretory organs in the heads of some crustaceans. Ark Zool 30 B 8:1–3
Zaffagnini F (1987) Reproduction in Daphnia. In: Peters RH, De Bernardi R (eds) Daphnia. Memoria dell’ Istituto Italiano di Idrobiologia Dott. Marco de Marchi vol 45. Verbania Pallanza, Italy: Istituto Italiano di Idrobiologia, pp 245–284
Koshida Y, Hiroki M (1980) Artemia as a multipurpose biomaterial for biology education. In: Persoone G, Sorgeloos PM, Roels OEJ (eds) The brine shrimp Artemia. Universa Press, Wetteren, pp 289–298
Fingerman M (1985) The physiology and pharmacology of crustacean chromatophores. Am Zool 25(1):233–252
Gard AL, Lenz PH, Shaw JR, Christie AE (2009) Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen Comp Endocrinol 160(3):271–287
Colbourne JK, Eads BD, Shaw J, Bohuski E, Bauer DJ, Andrews J (2007) Sampling Daphnia’s expressed genes: preservation, expansion and invention of crustacean genes with reference to insect genomes. BMC Genomics 8:217. doi:10.1186/1471-2164-8-217
Eads BD, Andrews J, Colbourne JK (2008) Ecological genomics in Daphnia: stress responses and environmental sex determination. Heredity 100(2):184–190. doi:10.1038/sj.hdy.6800999
Tatarazako N, Oda S (2007) The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 16(1):197–203. doi:10.1007/s10646-006-0120-2
Persoone G, Baudo R, Cotman M, Blaise C, Thompson KCl, Moreira-Santos M, Vollat B, Törökne A, Han T (2009) Review on the acute Daphnia magna toxicity test—evaluation of the sensitivity and the precision of assays performed with organisms from laboratory cultures or hatched from dormant eggs. Knowl Managt Aquatic Ecosyst 393:01
LeBlanc GA (2007) Crustacean endocrine toxicology: a review. Ecotoxicology 16(1):61–81
Barata C, Alanon P, Gutierrez-Alonso S, Riva MC, Fernandez C, Tarazona JV (2008) A Daphnia magna feeding bioassay as a cost effective and ecological relevant sublethal toxicity test for environmental risk assessment of toxic effluents. Sci Total Environ 405(1–3):78–86. doi:10.1016/j.scitotenv.2008.06.028
Sterba G (1957) Die neurosekretorischen Zellgruppen einiger Cladoceren (Daphnia pulex und magna, Simocephalus vetulus). Zool Jahrb Anat 76:303–310
Halcrow K (1969) Sites of presumed neurosecretory activity in Daphnia magna Straus. Can J Zool 47:575–577
Van den Bosch de Aguilar P (1972) Les charactéristiques tinctoriales des cellules neurosécrétrices chez Daphnia pulex (Crustacea:Cladocera). Gen Comp Endocrinol 18:140–145
Montagné N, Desdevises Y, Soyez D, Toullec JY (2010) Molecular evolution of the crustacean hyperglycemic hormone family in ecdysozoans. BMC Evol Biol 10. doi:10.1186/1471-2148-10–62
Zhang Q, Keller R, Dircksen H (1993) Immunocytochemical mapping of peptidergic neurons in the nervous system of Daphnia magna (Crustacea; Cladocera). In: Elsner N, Heisenberg M (eds) Genes-brain-behaviour. Thieme, Stuttgart, p 545
Zhang Q, Keller R, Dircksen H (1997) Crustacean hyperglycaemic hormone in the nervous system of the primitive crustacean species Daphnia magna and Artemia salina (Crustacea: Branchiopoda). Cell Tissue Res 287(3):565–576
Zhang Q, Dircksen H (1999) Immunocytochemical mapping of crustacean cardioactive peptide in the nervous system of Daphnia magna and Artemia salina (Crustacea: Cladocera, Anostraca). Comp Biochem Physiol 124A:S86
Dircksen H, Zhang Q (2006) Neuropeptide-containing neurons in the central nervous system of the waterflea Daphnia magna. Comp Biochem Physiol 143A:S118
Van Harreveld A (1936) A physiological solution for freshwater crustaceans. Proc Soc Exp Biol Med 34:428–432
Stefanini M, De Martino C, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173–174
Dircksen H, Webster SG, Keller R (1988) Immunocytochemical demonstration of the neurosecretory systems containing putative moult-inhibiting and hyperglycemic hormone in the eyestalk of brachyuran crustaceans. Cell Tissue Res 251:3–12
Boer HH, Schot LPC, Roubos EW, ter Maat A, Lodder JC, Reichelt D, Swaab DF (1979) ACTH-like immunoreactivity in two electronically coupled giant neurons in pond snail Lymnaea stagnalis. Cell Tissue Res 202:231–240
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Löhr J, Klein J, Webster SG, Dircksen H (1993) Quantification, immunoaffinity purification and sequence analysis of a pigment-dispersing hormone of the shore crab, Carcinus maenas (L.). Comp Biochem Physiol B 104(4):699–706
Honda T, Matsushima A, Sumida K, Chuman Y, Sakaguchi K, Onoue H, Meinertzhagen IA, Shimohigashi Y, Shimohigashi M (2006) Structural isoforms of the circadian neuropeptide PDF expressed in the optic lobes of the cricket Gryllus bimaculatus: immunocytochemical evidence from specific monoclonal antibodies. J Comp Neurol 499(3):404–421
Sternberger LA (1974) Immunocytochemistry. Prentice Hall Inc., Englewood Cliffs, NJ
Dircksen H, Müller A, Keller R (1991) Crustacean cardioactive peptide in the nervous system of the locust, Locusta migratoria: an immunocytochemical study on the ventral nerve cord and peripheral innervation. Cell Tissue Res 263:439–457
Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C (2006) Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 26(9):2531–2543. doi:10.1523/JNEUROSCI.1234-05.2006
Aramant R, Elofsson R (1976) Distribution of monoaminergic neurons in the nervous system of non-malacostracan crustaceans. Cell Tissue Res 166(1):1–24
Cunnington WA (1903) Studien an einer Daphnide, Simocephalus sima. Beiträge zur Kenntnis des Centralnervensystems und der feineren Anatomie der Daphniden. Jena Z Naturwiss 37:447–520
Leder H (1915) Untersuchungen über den feineren Bau des Nervensystems der Cladoceren. Arb Zool Inst Univ Wien u Triest 20:297–392
Binder G (1932) Das Muskelsystem von Daphnia. Int Rev ges Hydrobiol Hydrograph 26:54–111
Nässel DR, Elofsson R, Odselius R (1978) Neuronal connectivity patterns in the compound eyes of Artemia salina and Daphnia magna (Crustacea: Branchiopoda). Cell Tissue Res 190(3):435–437
Strausfeld NJ, Nässel DR (1980) Neuroarchitecture of brain regions that subserve the compound eyes of Crustacea and insects. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6b: comparative physiology and evolution of vision in invertebrates. Springer, Berlin Heidelberg New York, pp 1–133
Helfrich-Förster C (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337(2):177–190
Fernandez MP, Berni J, Ceriani MF (2008) Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol 6(3):e69. doi:10.1371/journal.pbio.0060069
Ohira T, Tsutsui N, Kawazoe I, Wilder MN (2006) Isolation and characterization of two pigment-dispersing hormones from the whiteleg shrimp, Litopenaeus vannamei. Zool Sci 23(7):601–606
Park JH, Hall JC (1998) Isolation and chronobiological analysis of a neuropeptide pigment-dispersing factor gene in Drosophila melanogaster. J Biol Rhythms 13(3):219–228
Rao KR, Riehm JP (1988) Pigment-dispersing hormones: a novel family of neuropeptides from arthropods. Peptides 1:153–159
Yang WJ, Aida K, Nagasawa H (1999) Characterization of chromatophorotropic neuropeptides from the kuruma prawn Penaeus japonicus. Gen Comp Endocrinol 114(3):415–424
Bulau P, Meisen I, Schmitz T, Keller R, Peter-Katalinic J (2004) Identification of neuropeptides from the sinus gland of the crayfish Orconectes limosus using nanoscale on-line liquid chromatography tandem mass spectrometry. Mol Cell Proteomics 3(6):558–564
Janssen T, Husson SJ, Meelkop E, Temmerman L, Lindemans M, Verstraelen K, Rademakers S, Mertens I, Nitabach M, Jansen G, Schoofs L (2009) Discovery and characterization of a conserved pigment-dispersing factor-like neuropeptide pathway in Caenorhabditis elegans. J Neurochem 111(1):228–241. doi:10.1111/j.1471-4159.2009.06323.x
Lehman N, Pfrender ME, Morin PA, Crease TJ, Lynch M (1995) A hierarchical molecular phylogeny within the genus Daphnia. Mol Phylogenet Evol 4(4):395–407. doi:10.1006/mpev.1995.1037
Webster SG (1998) Peptidergic neurons in barnacles: an immunohistochemical study using antisera raised against crustacean neuropeptides. Biol Bull 195(3):282–289
Sousa GL, Lenz PH, Hartline DK, Christie AE (2008) Distribution of pigment-dispersing hormone- and tachykinin-related peptides in the central nervous system of the copepod crustacean Calanus finmarchicus. Gen Comp Endocrinol 156(3):454–459. doi:10.1016/j.ygcen.2008.03.008
Mangerich S, Keller R, Dircksen H (1986) Immunocytochemical identification of structures containing putative red pigment-concentrating hormone in two species of decapod crustaceans. Cell Tissue Res 245:377–386
Yasuyama K, Meinertzhagen IA (2010) Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol 518(3):292–304. doi:10.1002/cne.22210
Sandeman DC, Wilkens LA (1983) Motor control of movements of the antennal flagellum in the Australian crayfish, Euastacus armatus. J Exp Biol 105:253–273
Angel MV (1967) A histological and experimental approach to neurosecretion in Daphnia magna. In: Stutinsky F (ed) Neurosecretion. Springer, Berlin Heidelberg New York, pp 230–237
Van den Bosch de Aguilar P (1969) Nouvelles données morphologiques et hypothèses sur le rôle du système neurosécréteur chez Daphnia pulex (Crustacea: Cladocera). Ann Soc Roy Zool Belg 99 (1–2):27–44
Retzius G (1906) Zur Kenntnis des Nervensystems der Daphniden. Biol Unters N F 13:107–116
Gorgels-Kallen JL, Voorter CEM (1985) The secretory dynamics of the CHH-producing cell group in the eyestalk of the crayfish, Astacus leptodactylus, in the course of the day/night cycle. Cell Tissue Res 241:361–366
Kallen JL, Abrahamse SL, van Herp F (1990) Circadian rhythmicity of the crustacean hyperglycemic hormone (CHH) in the hemolymph of the crayfish. Biol Bull 179:351–357
Fanjul-Moles ML, Escamilla-Chimal EG, Salceda R, Giulianini PG, Sanchez-Chavez G (2010) Circadian modulation of crustacean hyperglycemic hormone in crayfish eyestalk and retina. Chronobiol Int 27(1):34–51
Aréchiga H, Rodriguez-Sosa L (2002) Distributed circadian rhythmicity in the crustacean nervous system. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin Heidelberg New York, pp 113–122
Fanjul-Moles ML, Prieto-Sagredo J (2003) The circadian system of crayfish: a developmental approach. Microsc Res Tech 60:291–301
Dini ML, Carpenter SR (1992) Fish predators, food availability and diel vertical migration in Daphnia. J Plankton Res 14(3):359–377
Makino W, Haruna H, Ban S (1996) Diel vertical migration and feeding rhythm of Daphnia longispina and Bosmina coregoni in Lake Toya, Hokkaido, Japan. Hydrobiologia 337(1–3):133–143
Young S, Watt PJ (1996) Daily and seasonal vertical migration rhythms in Daphnia. Freshwater Biol 36(1):17–22
Ringelberg J (1999) The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biol Rev Cambridge Phil Soc 74(4):397–423
Rammner W (1929) Über periodische Erscheinungen am Cladoceren-Individuum (Häutung, Fortpflanzung, Wachstum). Internat Rev Ges Hydrobiol u Hydrograph 21(5/6):402–420
Sed’a J (1992) Diurnal periodicity in Daphnia reproduction. Hydrobiologia 246(2):119–127
Stross RG (1996) Significance of photoperiodism and diapause control in the multicycle crustacean Daphnia pulex Leydig. Hydrobiologia 320:107–117
Cellier-Michel S, Berthon JL (2003) Rhythmicity of the pigments in the compound eye of Daphnia longispina (Cladocera). J Freshw Ecol 18(3):445–450
Ringelberg J, Seruaas H (1971) A circadian rhythm in Daphnia magna. Oecologia 6(3):289–292
Helfrich-Förster C (1998) Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol [A] 182(4):435–453
Sehadova H, Sauman I, Sehnal F (2003) Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res 312:113–125
Helfrich-Förster C (2009) Neuropeptide PDF plays multiple roles in the circadian clock of Drosophila melanogaster. Sleep Biol Rhythms 7:130–143
Helfrich-Förster C (2003) The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech 62(2):94–102
McTaggart SJ, Conlon C, Colbourne JK, Blaxter ML, Little TJ (2009) The components of the Daphnia pulex immune system as revealed by complete genome sequencing. BMC Genomics 10:175. doi:10.1186/1471-2164-10-175
Fernlund P (1976) Structure of a light-adapting hormone from the shrimp, Pandalus borealis. Biochim Biophys Acta 439(1):17–25
Mohrherr CJ, Rao KR, Riehm JP (1991) Characterization of a pigment-dispersing factor from the American cockroach. Soc Neurosci Abstr 17:276
Acknowledgments
The Carl Tryggers Foundation, Stockholm, Sweden (to J.S. and H.D.), the Faculty of Sciences, Stockholm University (to H.D.), the Federal ministry for research and technology, Germany (BMFT, to Q.Z.), and postdoctoral research grants from the Fund for Scientific Research—Flanders, Belgium (F.W.O.-Vlaanderen, to P.V., J.H., K.P.), and the German Research Foundation (DFG to R.P.) are gratefully acknowledged by the authors. We are greatly indebted to Achim Stommel, State office for nature, environment, and customer protection, North-Rhine Westphalia (LaNUV), Bonn, Germany, for providing Daphnia magna and green algae stocks and many useful rearing advises. The authors also wish to thank Dr. S.G. Webster, University of North Wales, Bangor, UK for his generous gift of synthetic Uca-beta-PDH. The authors wish to thank our facility manager Dr. Stina Höglund, Wenner-Gren institute, Stockholm University, for technical help with confocal microscopy, and Dr. Anthony Poole, Department of Molecular Biology and Functional Genomics, Stockholm University, for help with final stylistical polishing of the text.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strauß, J., Zhang, Q., Verleyen, P. et al. Pigment-dispersing hormone in Daphnia interneurons, one type homologous to insect clock neurons displaying circadian rhythmicity. Cell. Mol. Life Sci. 68, 3403–3423 (2011). https://doi.org/10.1007/s00018-011-0636-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0636-3