Abstract
Objective
The cell line, RBL-2H3, has been widely used as a mast cell model though much of the data is contradictory. The aim of this study is to assess the RBL-2H3 cell line as an in vitro model for degranulation studies.
Methods
RBL-2H3 cells were stimulated with either dinitrophenylated-IgE, calcium ionophore A23187, compound 48/80, mast cell degranulating peptide or lipopolysaccharide and mediator (histamine, β-hexosaminidase, interleukin-13 and TNF-α) release was analysed. Toll-like receptors (TLR) mRNA expression in RBL-2H3 cells and rat peritoneal mast cells (RPMC) were compared by RT-PCR. TLR4 and CD14 surface proteins in RBL-2H3 were analysed by FACS.
Results
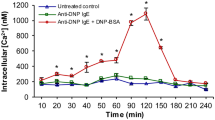
Mediator release was dependant on media composition and degranulation was stimulated by IgE and A23187. Degranulation was inhibited by quercetin, but not by cromoglycate or ketotifen. Transcripts encoding TLR3-5 and 6 were detected in RBL-2H3 cells whereas TLR1-6 and TLR8 were clearly seen in RPMC. While proteins were detected for TLR4 in RBL-2H3 cells, CD14 was largely absent.
Conclusions
While RBL-2H3 cells may be useful as a model for mast cell IgE-mediated degranulation, other aspects may not be representative and they may share similarities with basophils rather than with other histamine-releasing cell types.





Similar content being viewed by others
References
Puxeddu I, Piliponsky AM, Bachelet I, Levi-Schaffer F. Mast cells in allergy and beyond. Int J Biochem Cell Biol. 2003;35:1601–7.
Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–7.
Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–9.
Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, et al. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci USA. 1985;82:3871–5.
Saito H, Kato A, Matsumoto K, Okayama Y. Culture of human mast cells from peripheral blood progenitors. Nat Protoc. 2006;1:2178–83.
Coutts SM, Nehring RE Jr, Jariwala NU. Purification of rat peritoneal mast cells: occupation of IgE-receptors by IgE prevents loss of the receptors. J Immunol. 1980;124:2309–15.
Horigome K, Bullock ED, Johnson EM Jr. Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J Biol Chem. 1994;269:2695–702.
Siraganian RP, McGivney A, Barsumian EL, Crews FT, Hirata F, Axelrod J. Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed Proc. 1982;41:30–4.
Ortega E, Schweitzer-Stenner R, Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988;7:4101–9.
Funaba M, Ikeda T, Abe M. Degranulation in RBL-2H3 cells: regulation by calmodulin pathway. Cell Biol Int. 2003;27:879–85.
Ikawati Z, Wahyuono S, Maeyama K. Screening of several Indonesian medicinal plants for their inhibitory effect on histamine release from RBL-2H3 cells. J Ethnopharmacol. 2001;75:249–56.
Kobayashi M, Matsushita H, Yoshida K, Tsukiyama R, Sugimura T, Yamamoto K. In vitro and in vivo anti-allergic activity of soy sauce. Int J Mol Med. 2004;14:879–84.
Shore PA, Burkhalter A, Cohn VH Jr. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182–6.
Aketani S, Teshima R, Umezawa Y, Sawada J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol Lett. 2001;75:185–9.
Glaum MC, Wang Y, Raible DG, Schulman ES. Degranulation influences heparin-associated inhibition of RT-PCR in human lung mast cells. Clin Exp Allergy. 2001;31:1631–5.
Curzon G, Green AR. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970;39:653–5.
Buku A, Price JA, Mendlowitz M, Masur S. Mast cell degranulating peptide binds to RBL-2H3 mast cell receptors and inhibits IgE binding. Peptides. 2001;22:1993–8.
Konca K, Tiftik EN, Aslan G, Kanik A, Yalcin A. The effect of cromoglycate on time-dependent histamine and serotonin concentrations in stored blood products. Transfus Apher Sci. 2006;34:193–8.
Fox CC, Wolf EJ, Kagey-Sobotka A, Lichtenstein LM. Comparison of human lung and intestinal mast cells. J Allergy Clin Immunol. 1988;81:89–94.
Fernandez M, Cordoba H, Santos F, Oehling A. In vitro ketotifen protection in bronchial hyperreactivity. J Investig Allergol Clin Immunol. 1991;1:377–82.
Middleton E Jr, Drzewiecki G, Krishnarao D. Quercetin: an inhibitor of antigen-induced human basophil histamine release. J Immunol. 1981;127:546–50.
Dearman RJ, Skinner RA, Deakin N, Shaw D, Kimber I. Evaluation of an in vitro method for the measurement of specific IgE antibody responses: the rat basophilic leukemia (RBL) cell assay. Toxicology. 2005;206:195–205.
Coleman JW. A kinetic analysis of the in vitro sensitization of murine peritoneal mast cells with monoclonal IgE anti-DNP antibody. Immunology. 1988;64:527–31.
Gibbs BF, Rathling A, Zillikens D, Huber M, Haas H. Initial Fc epsilon RI-mediated signal strength plays a key role in regulating basophil signaling and deactivation. J Allergy Clin Immunol. 2006;118:1060–7.
Gon Y, Nunomura S, Ra C. Common and distinct signalling cascades in the production of tumour necrosis factor-alpha and interleukin-13 induced by lipopolysaccharide in RBL-2H3 cells. Clin Exp Allergy. 2005;35:635–42.
Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301.
Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45.
Eccleston E, Leonard BJ, Lowe JS, Welford HJ. Basophilic leukaemia in the albino rat and a demonstration of the basopoietin. Nat New Biol. 1973;244:73–6.
Buell DN, Fowlkes BJ, Metzher H, Isersky C. Cell cycle and morphological changes during growth and differentiation of a rat basophilic leukemia cell line. Cancer Res. 1976;36:3131–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: A. Falus.
Rights and permissions
About this article
Cite this article
Passante, E., Ehrhardt, C., Sheridan, H. et al. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm. Res. 58, 611–618 (2009). https://doi.org/10.1007/s00011-009-0028-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0028-4