Abstract
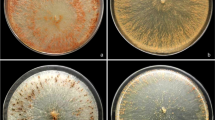
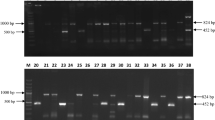
One of the major diseases affecting Hevea brasiliensis is the white root rot caused by the fungus Rigidoporus microporus. Current treatment measures taken to control rubber diseases are highly dependent on chemical fungicides which pollute the environment and pose health hazards to workers. The utilisation of chitinolytic microorganisms for disease control is an attractive alternative. A total of 61 chitinolytic isolates were isolated from soil and tested for antagonism against R. microporus using dual culture assay. Out of the 61 chitinolytic microorganisms, 46 exhibited inhibition against R. microporus ranging from 3.67% to 85.65% with isolate SPSB 4-4 recording the highest percentage inhibition. Light microscopy observation revealed degradation of mycelium implying the hydrolysis of fungal cell wall by chitinase. Nine chitinolytic microorganisms which exhibited the highest percent inhibition were selected for dipped stick inhibition assay. All nine isolates inhibited the growth of R. microporus with percent inhibition ranging from 31.69% to 91.63% with isolate SPSB 4-4 exhibiting the highest percent inhibition. These nine isolates were sent for 16S rRNA gene sequencing for bacterial identification. The isolates were identified to be from the genus Pseudomonas, Burkholderia and Streptomyces. These chitinolytic microorganisms hold great potential to be developed as effective, environmentally friendly and worker-safe solution for crop protection.
Similar content being viewed by others
References
JOHNSTON, A. (1989) Diseases and Pest. In: Webster, C.C., Baulkwill, W.J. (Eds), Rubber. New York: Longman Scientific and Technical, 415–458.
RAO, B. S. (1975) Maladies of Hevea in Malaysia. Kuala Lumpur: Rubber Research Institute of Malaysia.
RUBBER RESEARCH INSTITUTE MALAYSIA (1974) Root Diseases of Hevea (Editorial) Planters’ Bulletin, 133, 109–110.
GOHEL, V., SINGH, A., VIMAL, M., ASHWINI, P. AND CHHATPAR, H.S. (2006) Bioprospecting and antifungal potential of chitinolytic microorganisms. Afr J Biotechnol, 5(2), 54–72.
SIVAN, A. AND CHET, I. (1989) Degradation of Fungal Cell Walls by Lytic Enzymes of Trichoderma harzianum. J. Gen. Microbiol., 135(3), 675–682.
IDWAN SUDIRMAN, L., IRAQI HOUSSEINI, A.I., LE FEBVRE, G., KIFFER, E. AND BOTTON, B. (1992) Screening of Some Basidiomycetes for Biocontrol of Rigidoporus microporus, A Parasite of the Rubber Tree Hevea brasiliensis. Mycological Research, 96(8), 621–625.
JAYASURIYA, K.E., AND DEACON, J.W. (1995) In Vitro Interactions between Rigidoporus microporus, the Cause of White Root Disease of Rubber and Some Potentially Antagonistic Fungi. J. Rubb. Res. Inst. Sri Lanka, 76, 36–54.
JAYASURIYA, K.E. AND THENNAKOON, B.I. (2007) Biological Control of Rigidoporus microporus, the Cause of White Root Disease in Rubber. Cey. J. Sci. (Bio. Sci.), 36(1), 9–16.
KAEWCHAI, S. AND SOYTONG, K. (2010) Application of Biofungicides Against Rigidoporus microporus Causing White Root Disease of Rubber Trees. J. Agric. Technol., 6(2), 349–363.
SHARMA, N., SHARMA, K.P., GAUR, R.K., AND GUPTA, V.K. (2011) Role of Chitinase in Plant Defense. Asian Journal of Biochemistry, 6(1), 29–37.
NEERAJA, C., ANIL, K., PURUSHOTHAM, P., SUMA, K., SARMA, P.V.S.R.N., MOERSCHBACHER, B.M., AND PODILE, A.R. (2010) Biotechnological Approaches to Develop Bacterial Chitinases as a Bioshield against Fungal Diseases of Plants. Critical Reviews in Biotechnology, 30(3), 231–241.
NAGPURE, A., CHOUDHARY, B., KUMAR, S., AND GUPTA, R.K. (2013) Isolation and Characterization of Chitinolytic Streptomyces sp. MT7 and Its Antagonism towards Wood-Rotting Fungi. Ann. Microbiol. 64 (2), 531–541.
PATIL, R.S., GHORMADE, V.V., AND DESHPANDE, M.V. (2000) Chitinolytic Enzymes: An Exploration. Enzyme Microb. Technol., 26(7), 473–483.
REN, Y.Y., AND WEST, C.A. (1992) Elicitation of Diterpene Biosynthesis in Rice (Oryza sativa L.) by Chitin. Plant Physiol, 99(3), 1169–1178.
MANJULA, K., KISHORE, G.K. AND PODILE, A.R. (2004) Whole Cells of Bacillus subtilis AF 1 Proved More Effective Than Cell-Free and Chitinase-Based Formulations in Biological Control of Citrus Fruit Rot and Groundnut Rust. Can. J. Microbiol., 50(9), 737–744.
KISHORE, G.K., PANDE, S., AND PODILE, A.R. (2005) Biological Control of Late Leaf Spot of Peanut (Arachis hypogaea) with Chitinolytic Bacteria. Phytopathology, 95(10), 1157–1165.
AJIT, N. S., VERMA, R. AND SHANMUGAM, V. (2006) Extracellular Chitinases of Fluorescent Pseudomonads Antifungal to Fusarium oxysporum f. sp. Dianthi Causing Carnation Wilt. Current Microbiology, 52(4), 310–316.
MUKHERJEE, G., AND SEN, S.K. (2006) Purification, Characterization, and Antifungal Activity of Chitinase from Streptomyces venezuelae P10. Current Microbiology, 53(4), 265–269.
YANG, C. Y., HO, Y.C., PANG, J.C., HUANG, S.S., AND TSCHEN, J.S.M. (2009) Cloning and Expression of an Antifungal Chitinase Gene of a Novel Bacillus subtilis Isolate from Taiwan Potato Field. Bioresource Technology, 100(3), 1454–1458.
ANITHA, A. AND RABEETH, M. (2010) Degradation of Fungal Cell Walls of Phytopathogenic Fungi by Lytic Enzyme of Streptomyces griseus. African Journal of Plant Science, 4(3), 61–66.
CHANG, W.T., CHEN, M.L., AND WANG, S.L. (2010) An Antifungal Chitinase Produced by Bacillus subtilis Using Chitin Waste as a Carbon Source. World J Microbiol. Biotechnol., 26(5), 945–950.
LIU, D., CAI, J., XIE, C. C., LIU, C. AND CHEN, Y. H. (2010) Purification and Partial Characterization of a 36-kDa Chitinase from Bacillus thuringiensis subsp. colmeri, and its Biocontrol Potential. Enzyme and Microbial Technology, 46(3), 252–256.
ABDEL-SHAKOUR, E.H. (2012) Assessment of Antifungal Activity of Chitinase Produced by Bacillus licheniformis EG5 Isolated from Egyptian Soil. Life Science Journal, 9(4), 3560–3572.
BRZEZINSKA, M.S., AND JANKIEWICZ, U. (2012) Production of Antifungal Chitinase by Aspergillus niger LOCK 62 and Its Potential Role in the Biological Control. Curr. Microbiol., 65(6), 666–672.
EMAN ZAKARIA, G. (2012) Chitinase Production by Bacillus thuringiensis and Bacillus licheniformis: Their Potential in Antifungal Biocontrol. The Journal of Microbiology, 50(1), 103–111.
JANKIEWICZ, U. BRZEZINSKA, M.S., AND SAKS, E. (2012) Identification and Characterization of a Chitinase of Stenotrophomonas maltophilia, A Bacterium that is Antagonistic Towards Fungal Phytopthogens. Journal of Bioscience and Bioengineering, 113(1), 30–35.
JIANG, X., CHEN, D., HONG, S., WANG, W., CHEN, S., AND ZOU, S. (2012) Identification, Characterization and Functional Analysis of a GH-18 Chitinase from Streptomyces roseolus. Carbohydrate Polymers, 87(4), 2409–2415.
KUMAR, D.P., KUMAR SINGH, R., ANUPAMA, P.D., SOLANKI, M.K., KUMAR, S., SRIVASTAVA, A.K., SINGHAL, P.K., AND ARORA, D.K. (2012) Studies on Exo-Chitinase Production from Trichoderma asperellum UTP-16 and Its Characterization. Indian J. Microbiol, 52(3), 388–395.
SOLANKI, M.K., ROBERT, A.S., SINGH, R.K., KUMAR, S., PANDEY, A.K., SRIVASTAVA, A.K. AND ARORA, D.K. (2012) Characterization of Mycolytic Enzymes of Bacillus Strains and Their Bio-Protection Role against Rhizoctonia solani in Tomato. Curr. Microbiol., 65(3), 330–336.
PATIL, N.S., WAGHMARE, S.R., AND JADHAV, J.P. (2013) Purification and Characterization of an Extracellular Antifungal Chitinase from Penicillium ochrochloron MTCC 517 and Its Application in Protoplast Formation. Process Biochemistry, 48(1), 176–183.
PRASANNA, L., EIJSINK, V.G.H., MEADOW, R., AND GASEIDNES, S. (2013) A Novel Strain of Brevibacillus laterosporus Produces Chitinases that Contribute to Its Biocontrol Potential. Appl. Microbiol. Biotechnol., 97(4), 1601–1611.
SUMA, K., AND PODILE, A. R. (2013) Chitinase A from Stenotrophomonas maltophilia Shows Transglycosylation and Antifungal Activities. Bioresource Technology, 133, 213–220.
WANG, S.Y. ZHOU, J.J., SHAO, B., LU, Y.J., AND RAO, P.F. (2008) A Thermostable Chitinase with Chitin-Binding Activity from Phaseolus limensis. Journal of Food Science, 73(6), 452–457.
WANG, S., SHAO, B., FU, H., AND RAO, P. (2009) Isolation of a Thermostable Legume Chitinase and Study on the Antifungal Activity. Appl. Microbiol. Biotechnol., 85(2), 313–321.
WANG, S., YE, X., CHEN, J., AND RAO, P. (2012) A Novel Chitinase Isolated from Vicia faba and Its Antifungal Activity. Food Research International, 45(1), 116–122.
ZHANG, J., KOPPARAPU, N.K., YAN, Q., YANG, S., AND JIANG, Z. (2013) Purification and Characterisation of a Novel Chitinase from Persimmon (Diospyros kaki) with Antifungal Activity. Food Chemistry, 138(2–3), 1225–1232.
BRZEZINSKA, M.S., JANKIEWICZ, U., BURKOWSKA, A. AND WALCZAK, M. (2014) Chitinolytic Microorganisms and Their Possible Application in Environmental Protection. Curr. Microbiol., 68(1), 71–81.
EL-TARABILY, K.A., SOLIMAN, M.H., NASSAR, A.H., AL-HASSANI, H.A., SIVASITHAMPARAM, K., MCKENNA, F. AND HARDY, G.E.ST.J. (2000) Biological Control of Sclerotinia Minor Using a Chitinolytic Bacterium and Actinomycetes. Plant Pathology, 49(5), 573–583.
KAMIL, Z., RIZK, M., SALEH, M. AND MOUSTAFA, S. (2007) Isolation and Identification Of Rhizosphere Soil Chitinolytic Bacteria and Their Potential in Antifungal Biocontrol. Global Journal of Molecular Sciences, 2(2), 57–66.
HARIPRASAD, P., DIVAKARA, S. T. AND NIRANJANA, S. R. (2011) Isolation and Characterization of Chitinolytic Rhizobacteria for the Management of Fusarium Wilt in Tomato. Crop Protection, 30(2), 1606–1612.
TANG-UM, J. AND NIAMSUP, H. (2012) Chitinase Production and Antifungal Potential of Endophytic Streptomyces Strain P4. Maejo International Journal of Science and Technology, 6(1), 95–104.
PATTANAPIPITPAISAL, P. AND KAMLANDHARN, R. (2012) Screening of Chitinolytic Actinomycetes for Biological Control of Sclerotium rolfsii Stem Rot Disease of Chilli. Songklanakarin Journal of Science and Technology, 34(4), 387–393.
WANG, K., YAN, P.S., CAO, L.X., DING, Q.L., SHAO, C. AND ZHAO, T.F. (2013) Potential of Chitinolytic Serratia marcescens Strain JPP1 For Biological Control Of Aspergillus parasiticus and Aflatoxin. BioMed Research International. https://doi.org/10.1155/2013/397142
MURTHY, N. AND BLEAKLEY, B. (2012) Simplified method of preparing colloidal chitin used for screening of chitinase-producing microorganisms. The Internet Journal of Microbiology, 10(2).
MEENA, B., MARIMUTHU, T., VIDHYASEKARAN, P. AND VELAZHAHAN, R. (2001) Biological Control of Root Rot of Groundnut with Antagonistic Pseudomonas fluorescens Strains. J. Plant Dis. Protect., 108(4), 368–381.
PARK, M.S., JUNG, S.R., LEE, M.S., KIM K.O., DO, J.O., LEE, K.H., KIM, S.B., AND BAE, K.S. (2005). Isolation and Characterization of Bacteria Associated with Two Sand Dune Plant Species, Calystegia soldanella and Elymus mollis. J. Microbiol. 43(3), 219–227.
JANDA, J.M. AND ABBOTT, S.L. (2007) 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils and Pitfalls. Journal of Clinical Microbiology, 45(9), 2761–2764.
WELLER, D.M. (2007) Pseudomonas Biocontrol Agents of Soilborne Pathogens: Looking Back Over 30 Years. Phytopathology, 97(2), 250–256.
VISWANATHAN, R. AND SAMIYAPPAN, R. (2002) Induced Systemic Resistance By fluorescent Pseudomonads against Red Rot Disease of Sugarcane Caused by Colletotrichum falcatum. Crop Protection, 21(1), 1–10.
SALAHEDDIN, K., VALLUVAPARIDASAN, V., LADHALAKSHMI, D. AND VELAZHAHAN, R. (2010) Management of Bacterial Blight of Cotton Using A Mixture of Pseudomonas fluorescens and Bacillus subtilis. Plant Protect. Sci., 46(2), 41–50.
SHIRZAD, A., FALLAHZADEH-MAMAGHANI, V. AND PAZHOUHANDEH, M. (2012) Antagonistic Potential of Fluorescent Pseudomonads and Control of Crown and Root Rot of Cucumber Caused by Phythophtora drechsleri. Plant Pathol. J., 28(1), 1–9.
KHANUCHIYA, S., PARABIA, F.M., PATEL, M., PATEL, V., PATEL, K. AND GAMI, B. (2012) Effect of Pseudomonas fluorescence, P. aeruginosa and Bacillus subtilis As Biocontrol Agent For Crop Protection. Cibtech Journal of Microbiology, 1(1), 52–59.
COENYE, T., MAHENTHIRALINGAM, E., HENRY, D., LIPUMA, J.J., LAEVENS, S., GILLIS, M., SPEERT, D.P. AND VANDAMM, P. (2003) Burkholderia ambifaria sp. nov., A Novel Member of the Burkholderia cepacia Complex Including Biocontrol and Cystic fibrosis-Related Isolates. International Journal of Systematic and Evolutionary Microbiology, 51, 1481–1490.
ROBERTS, D.P., LOHRKE, S.M., MEYER, S.L.F., BUYER, J.S. BOWERS, J.H., BAKER, C.J., LI, W., DE SOUZA, J.T., LEWIS, J.A. AND CHUNG, S. (2005) Biocontrol Agents Applied Individually and in Combination for Suppression of Soilborne Diseases of Cucumber. Crop Protection, 24(2), 141–155.
SCUDERI, G., BONACCORSI, A., PANEBIANCO, S., VITALE, A., POLIZZI, G. AND CIRVILLERI, G. (2009) Some Strains of Burkholderia gladioliare Potential Candidates for Postharvest Biocontrol of Fungal Rots in Citrus and Apple Fruits. Journal of Plant Pathology, 91(1), 207–213.
AZADEH, B.F., SARIAH, M. AND WONG, M.Y. (2010) Characterization of Burkholderia cepacia Genomovar I as a Potential Biocontrol Agent of Ganoderma boninense in Oil Palm. African Journal of Biotechnology, 9(24), 3542–3548.
SATYA, V.K., VIJAYASAMUNDEESWARI, A., PARANIDHARAN, V. AND VELAZHAHAN, R. (2011) Burkholderia sp. Strain TNAU-1 for Biological Control of Root Rot in Mung Bean (Vigna radiata L.) Caused by Macrophomina phaseolina. Journal of Plant Protection Research, 51(3), 273–278.
DEVI, S.I., SOMKUWAR, B., POTSHANGBAM M. AND TALUKDAR, N.C. (2012) Genetic Characterization of Burkholderia cepacia Strain from Northeast India: A Potential Bio-Control Agent. Advances in Bioscience and Biotechnology, 3(8), 1179–1188.
DE LOS SANTOS-VILLALOBOS, S., BARRERA-GALICIA, G.C., MIRANDA-SALCEDO, M.A. AND PENA-CABRIALES, J.J. (2012) Burkholderia cepacia XXVI Siderophore with Biocontrol Capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol., 28(8), 2615–2623.
ERRAKHI, R., BOUTEAU, F., LEBRIHI, A. AND BARAKATE, M. (2007) Evidences of Biological Control Capacities of Streptomyces spp. against Sclerotium rolfsii Responsible for Damping-off Disease in Sugar Beet (Beta vulgaris L.). World J. Microbiol. Biotechnol., 23(11), 1503–1509.
SOUSA, C.D.S., FERMINO SOARES, A.C.F. AND GARRIDO, M.D.S. (2008) Characterization of Streptomycetes with Potential to Promote Plant Growth and Biocontrol. Sci. Agric., 65(1), 50–55.
SHTERNSHIS, M.V., BELJAEV, A.A., SHPATOVA, T.V., BOKOVA, J.V. AND DUZHAK, A.B. (2002) Field Testing of Bacticide, Phytoverm and Chitinase for Control of the Raspberry Midge Blight in Siberia. BioControl, 47(6), 697–706.
PRAPAGDEE, B., KUEKULVONG, C. AND MONGKOLSUK, S. (2008) Antifungal Potential of Extracellular Metabolite Produced by Streptomyces hygroscopicus Against Phytopathogenic Fungi. Int. J. Biol. Sci., 4(5), 330–337.
QUECINE, M.C. ARAUJO, W.L., MARCON, J. GAI, C.S., AZEVEDO J.L. AND PIZZIRANI-KLEINER, A.A. (2008) Chitinolytic Activity of Endophytic Streptomyces and Potential for Biocontrol. Letters in Applied Microbiology, 47(6), 486–491.
SAJITHA, K.L. AND FLORENCE, E.J.M. (2013) Effects of Streptomyces sp. on Growth of Rubberwood Sapstain Fungus Lasiodiplodia theobromae. Journal of Tropical Forest Science, 25(3), 393–399.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maiden, N.A., Noran, A.S., Ahmad Fauzi, M.A.F. et al. Screening and Characterisation of Chitinolytic Microorganisms with Potential to Control White Root Disease of Hevea brasiliensis. J Rubber Res 20, 182–202 (2017). https://doi.org/10.1007/BF03449151
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03449151