Abstract
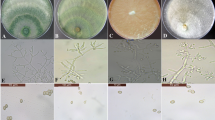
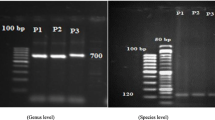
Thirty-eight isolates of Trichoderma spp. established from the rhizosphere of sugarcane were characterized using a multiplex PCR assay and further assessed for the production of hydrolytic enzymes chitinase and cellulase. The results of multiplex PCR assay successfully identified 29 isolates and revealed T. harzianum to be the predominant species (21 isolates) in sugarcane rhizosphere followed by T. longibrachiatum (8 isolates). In enzymatic assays, chitinase production was recorded in 18 isolates and cellulase production observed in 17 isolates. However, there was a considerable variability in both chitinase and cellulase production potential across the isolates. Three T. longibrachiatum isolates (STr-52, STr-83 and STr-108) exhibited high production of both chitinase and cellulase. Talc formulation of two promising isolates (STr-83 and STr-108) was prepared and evaluated in the field conditions for their potential to suppress red rot under three different delivery systems, viz. sett treatment, soil application and their combination. All Trichoderma treatments resulted in considerable reduction in red rot (29.5–56.3%) over untreated control. However, the level of reduction accorded was much higher when the Trichoderma isolates were applied as a combination of sett and soil treatment (> 53% reduction) as compared to soil application (43–49% reduction) or sett treatment alone (< 35% reduction). Delivery of Trichoderma isolates as a combination of sett and soil treatment also exhibited highest NMCs and yield over untreated control. The application methods of talc formulations of these two isolates offer a feasible and effective option for large-scale application of these isolates for the management of red rot disease in sugarcane growing regions.

Similar content being viewed by others
References
Agrawal, T., and A.S. Kotasthane. 2012. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chattisgarh in central India. Springer Plus 1: 73.
Benoliel, B., F.A.G. Torres, and L.M.P. de Moraes. 2013. A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. Springer Plus 2: 656. https://doi.org/10.1186/2193-1801-2-656.
Bradner, J.R., M. Gillings, and K.M.H. Nevalainen. 1999. Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World Journal of Microbiology & Biotechnology 15: 131–132.
De Marco, J.L., L.H.C. Lima, M.V. de Sousa, and C.R. Felix. 2000. A Trichoderma harzianum chitinase destroys the cell wall of the phytopathogen Crinipellis perniciosa, the causal agent of witches’ broom disease of cocoa. World Journal of Microbiology & Biotechnology 16: 383–386. https://doi.org/10.1023/A:1008964324425.
Duttamajumder, S.K., and S.C. Misra. 2014. Dynamics of red rot (Colletotrichum falcatum) development and spread in relation to sett-borne inoculum. In: Proceedings of international conclave on sugar crops: sweeteners and green energy from sugar crops: emerging technology, IISR, Lucknow, Feb. 15–17, pp. 128.
Duttamajumder, S.K. 2008. Red rot of Sugarcane. Lucknow: Army Printing Press.
El-Katatny, M., M. Gudelj, K.H. Robra, M.A. Elnaghy, and G.M. Gubitz. 2001. Characterization of a chitinase and an endo-β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Applied Microbiology and Biotechnology 56: 137. https://doi.org/10.1007/s002530100646.
FAOSTAT. 2014. Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/567/default.aspx#ancor.
Freeman, S., D. Miz, I. Kolesnik, O. Barbul, A. Zveibil, M. Maymon, Y. Nitzani, B. Krihshner, D. Rav-David, A. Bilu, A. Dag, S. Shafir, and Y. Elad. 2004. Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. European Journal of Plant Pathology 110: 361–370.
Gajera, H.P., and D.N. Vakharia. 2010. Molecular and biochemical characterization of Trichoderma isolates inhibiting a phytopathogenic fungi Aspergillus niger Van Tieghem. Physiological and Molecular Plant Pathology 74: 274–282.
Harman, G.E. 2011. Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytologist 189: 647–649.
Hermosa, M.R., E. Keck, I. Chamorro, B. Rubio, L. Sanz, J.A. Vizcaino, I. Grondona, and E. Monte. 2004. Genetic diversity shown in Trichoderma biocontrol isolates. Mycological Research 108: 897–906.
Howell, C.R. 2003. Mechanisms employed by Trichoderma species in biological control of plant diseases: The history and evolution of current concepts. Plant Disease 87: 4–10.
Hoyos-Carvajal, L., S. Orduz, and J. Bissett. 2009. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genetics and Biology 46: 615–631.
Jain, N., A. Bhatia, and H. Pathak. 2014. Emission of air pollutants from crop residue burning in India. Aerosol Air Quality Research 14: 422–430.
Joshi, Deeksha, P. Singh, A.K. Singh, R.J. Lal, and Nidhi Tripathi. 2016. Antifungal potential of metabolites from Trichoderma sp. against Colletotrichum falcatum Went causing red rot of Sugarcane. Sugar Tech 18: 529–536. https://doi.org/10.10007/s12355-015-0421-y.
Joshi, Deeksha, and S.C. Misra. 2013. Characterization of Trichoderma isolates from sugarcane agro-ecosystem and their efficacy against Colletotrichum falcatum causing red rot of sugarcane. Sugar Tech 15: 192–196.
Kovacs, K., G. Szakacs, T. Pusztahelyi, and A. Pandey. 2004. Production of chitinolytic enzymes with Trichoderma longibrachiatum IMI 92027 in solid substrate fermentation. Applied Biochemistry and Biotechnology 118: 189. https://doi.org/10.1385/ABAB:118:1-3:189.
Kubicek, C.P., J. Bisset, I. Druzhinia, C.M. Kullnig-Gradinger, and G. Szakacs. 2003. Genetic and metabolic diversity of Trichoderma: a case study on South East Asian isolates. Fungal Genetics and Biology 38: 310–319.
Kubicek, C.P., M. Mikus, A. Schuster, M. Schmoll, and B. Seiboth. 2009. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnology Biofuels 2: 19.
Lopez-Quintero, C.A., L. Atanasova, A.E. Franco-Molano, W. Gams, M. Komon-Zelazowska, B. Theelen, W.H. Muller, T. Boekhout, and I. Druzhinina. 2013. DNA barcoding survey of Trichoderma diversity in soil and litter of the Colombian lowland Amazonian rainforest reveals Trichoderma strigosellum sp. nov. and other species. Antonie van Leeuwenhoek 104: 657–674.
Lopes, F.A.C., A.S. Steindorff, A.M. Geraldine, R.S. Brandao, V.N. Monteiro, M.L. Junior, A.S.G. Coelho, C.J. Ulhoa, and R.N. Silva. 2012. Biochemical and metabolic profiles of Trichoderma strains isolated from common bean crops in the Brazilian Cerrado, and potential antagonism against Sclerotinia sclerotiorum. Fungal Biology 116: 815–824.
Lorito, M., C. Peterbauer, C.K. Hayes, and G.E. Harman. 1994. Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 140: 623–629.
Lorito, M., S.L. Woo, M. D’Ambrosio, G.E. Harman, C.K. Hayes, C.P. Kubicek, and F. Scala. 1996. Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Molecular Plant-Microbe Interaction 9: 206–213.
Mulaw, T.B., C.P. Kubicek, and I.S. Druzhinina. 2010. The rhizosphere of Coffea arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity 2: 527–549. https://doi.org/10.3390/d2040527.
Prabhakaran, N., T. Prameeladevi, M. Sathiyabama, and D. Kamil. 2015. Multiplex PCR for detection and differentiation of diverse Trichoderma species. Annals of Microbiology 65: 1591–1595.
Romaro-Dumaresq, A.S., M.N. Dourado, L.C.L. Fávaro, R. Mendes, A. Ferreira, and W.L. Araújo. 2016. Diversity of cultivated fungi associated with conventional and transgenic sugarcane and the interaction between endophytic Trichoderma virens and the host plant. PLoS ONE. https://doi.org/10.1371/journal.pone.0158974.
Sánchez, V., O. Rebolledo, R.M. Picaso, E. Cárdenas, J. Córdova, O. González, and G.J. Samuels. 2007. In vitro antagonism of Thielaviopsis paradoxa by Trichoderma longibrachiatum. Mycopathologia 163: 49–58.
Singh, V., B.B. Joshi, S.K. Awasthi, and S.N. Srivastava. 2008. Eco-friendly management of red rot disease of sugarcane with Trichoderma strains. Sugar Tech 10: 158–161.
Singh, V., R.J. Lal, S.K. Awasthi, and M.R. Verma. 2009. Managing red rot of sugarcane by Trichoderma harzianum. Indian Sugar 59: 25–30.
Vinale, F., K. Sivasithamparam, E.L. Ghisalberti, R. Marra, S.L. Woo, and M. Lorito. 2008. Trichoderma-plant—pathogen interactions. Soil Biology & Biochemistry 40: 1–10.
Viswanathan, R., A. Ramesh Sundar, and S.M. Premkumari. 2003. Mycolytic effect of extracellular enzymes of antagonistic microbes to Colletotrichum falcatum, red rot pathogen of sugarcane. World Journal Microbiology and Biotechnology 19: 953–959.
Zhang, C., I.S. Druzhinina, C.P. Kubicek, and T. Xu. 2005. Trichoderma biodiversity in China: Evidence for a north to south distribution of species in east Asia. FEMS Microbiology Letters 251: 251–257.
Acknowledgements
We are grateful to the Director, ICAR-IISR, Lucknow, for providing facilities and constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Joshi, D., Singh, P., Holkar, S.K. et al. Trichoderma-Mediated Suppression of Red Rot of Sugarcane Under Field Conditions in Subtropical India. Sugar Tech 21, 496–504 (2019). https://doi.org/10.1007/s12355-018-0624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0624-0