Abstract
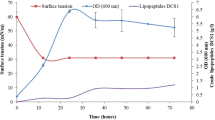
Colletotrichum gloeosporioides is the causal agent of anthracnose in mango. Burkholderia cepacia XXVI, isolated from mango rhizosphere and identified by 16S rDNA sequencing as a member of B. cepacia complex, was more effective than 6 other mango rhizosphere bacteria in inhibiting the model mango pathogen, C. gloeosporioides ATCC MYA 456. Biocontrol of this pathogen was demonstrated on Petri-dishes containing PDA by > 90 % reduction of surface colonization. The nature of the biocontrol metabolite(s) was characterized via a variety of tests. The inhibition was almost exclusively due to production of agar-diffusible, not volatile, metabolite(s). The diffusible metabolite(s) underwent thermal degradation at 70 and 121 °C (1 atm). Tests for indole acetic acid production and lytic enzyme activities (cellulase, glucanase and chitinase) by B. cepacia XXVI were negative, indicating that these metabolites were not involved in the biocontrol effect. Based on halo formation and growth inhibition of the pathogen on the diagnostic medium, CAS-agar, as well as colorimetric tests we surmised that strain XXVI produced a hydroxamate siderophore involved in the biocontrol effect observed. The minimal inhibitory concentration test showed that 0.64 μg ml−1 of siderophore (Deferoxamine mesylate salt-equivalent) was sufficient to achieve 91.1 % inhibition of the pathogen growth on Petri-dishes containing PDA. The biocontrol capacity against C. gloeosporioides ATCC MYA 456 correlated directly with the siderophore production by B. cepacia XXVI: the highest concentration of siderophore production in PDB on day 7, 1.7 μg ml−1 (Deferoxamine mesylate salt-equivalent), promoted a pathogen growth inhibition of 94.9 %. The growth of 5 additional strains of C. gloeosporioides (isolated from mango “Ataulfo” orchards located in the municipality of Chahuites, State of Oaxaca in Mexico) was also inhibited when confronted with B. cepacia XXVI. Results indicate that B. cepacia XXVI or its siderophore have the potential to be used as a biological control agent against C. gloeosporioides; thus diminishing environmental problems caused by the current practices to control this disease.




Similar content being viewed by others
References
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 45:12–39. doi:10.1007/BF00369386
Ann PJ, Chen MF, Hwang RC (1997) Effects of environmental factors on disease incidence of mango anthracnose and bacterial black spot. In: Tu CC, Yang CM (eds) Proceedings of the symposium on climatic effects on the occurrence of plant diseases and insects. Society of Agrometereology, Wufeng, Taichung, Taiwan, R.O.C, pp 29–40
Arias B, Carrizales L (2007) Control químico de la antracnosis del mango (Mangifera indica L.) en pre y postcosecha en el municipio Cedeño, estado Monagas, Venezuela. Bioagro 19(1):19–25
Arnow LE (1937) Colorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixtures. J Biol Chem 118:531–537
Arora NK, Kang SC, Maheshwari DK (2001) Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81:673–677
Avilán RL (2008) Nutrición y fertilización del mango. Int Plant Nutr Inst 33:44
Bernal G, Illanes A, Ciampi L (2002) Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp. with antibiotic activity against plant pathogenic agents. EJB 5:12–20
Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L, Belli ML, Piana S, Materazzo A, Vandamme P, Manno G (2002) Burkholderia cepacia complex bacteria from clinical and environmental sources in Italy: genomovar status and distribution of traits related to virulence and transmissibility. J Clin Microbiol 40:846–851. doi:10.1128/JCM.40.3.846-851
Carrillo-Castañeda G, Juárez-Muñoz J, Peralta-Videa J (2005) A spectrophotometric method to determine the siderophore production by strains of fluorescent Pseudomonas in the presence of copper and iron. Microchem J 81:35–40
Cartwright DK, Benson DM (1995) Comparison of Pseudomonas species and application techniques for biocontrol of Rhizoctonia stem rot of poinsettia. Plant Dis 79:309–313. doi:10.1094/PD-79-0309
Cartwright DK, Chilton C, Benson DM (1995) Pyrrolnitrin and phenazine production by Pseudomonas cepacia, strain 5.5B, a biological agent of Rhizoctonia solani. Appl Microbiol Biotechnol 43:211–216. doi:10.1007/BF00172814
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25:1919–1928. doi:10.1007/s11274-009-0090-7
Chen Y, Jurkevitch E, Bar-Ness E, Hadar Y (1994) Stability constants of pseudobactin complexes with transition metals. Soil Sci Soc Am J 58:390–396
Coenye T, Vandamme P, Govan JRW, Lipuma JJ (2001) Taxonomy and identification of the Burkholderia cepacia complex. JCM 39(10):3427–3436
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959. doi:10.1128/AEM
Cowart RE (2002) Reduction of iron by extracellular iron reductases: implications for microbial iron acquisition. Arch Biochem Biophys 400:273–281. doi:10.1016/S0003-9861(02)00012-7
Crosa JH (1997) Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev 61:319–336
Cuervo-Parra JA, Ramírez-Suero M, Sánchez-López V, Ramírez-Lepe M (2011) Antagonistic effect of Trichoderma harzianum VSL291 on phytopathogenic fungi isolated from cocoa (Theobroma cacao L.) fruits. Afr J Biotechnol 10(52):10657–10663
de los Santos-Villalobos S, de-Folter S, Délano-Frier JP, Gómez-Lim MA, Guzmán-Ortiz DA, Sánchez-García P, Peña-Cabriales JJ (2011) Puntos críticos en el manejo integral de mango: floración, antracnosis y residuos industriales. Rev Mex Cienc Agríc 2(2):221–234
Díaz de Villegas ME, Villa P, Frías A (2002) Evaluation of the siderophores production by Pseudomonas aeruginosa PSS. Rev Latinoam Microbiol 44:112–117
Drechsel H, Jung G (1998) Peptide siderophores. J Pept Sci 4:147–181
Eberl HJ, Collinson S (2009) A modeling and simulation study of siderophore mediated antagonism in dual-species biofilms. TBioMed 6(30):1–16
Estrada-de los santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. App Environ Microbiol 67(6):2790–2798
Ezziyyani M, Pérez C, Requena M, Ahmed A, Candela M (2004) Evaluación del biocontrol de Phytophthora capsici en pimiento (Capsicum annuum L.) por tratamiento con Burkholderia cepacia. Anales de Biología 26:61–68
Faraldo-Gómez JD, Sansom MSP (2003) Acquisition of siderophores in Gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116. doi:10.1038/nrm1015
Fischbach MA, Lin H, Liu DR, Walsh CT (2006) How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol 2:132–138
Fridlender M, Inbar J, Chet I (1993) Biological control of soilborne plant pathogens by a β-1,3 glucanase-producing Pseudomonas cepacia. Soil Biol Biochem 25:1211–1221. doi:10.1016/0038-0717(93)90217
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
González-Carreró MI, Sangari FJ, Agüero J, García-Lobo JM (2002) Brucella abortus strain 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiol 148:353–360
Harrison F, Paul J, Massey RC, Buckling A (2008) Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J 2(1):49–55
Hebbar KP, Martel MH, Heulin T (1998) Suppression of pre and post emergence damping off in corn by Burkholderia cepacia. Euro J Plant Pathol 104:29–36. doi:10.1023/A:1008625511924
Hwang J, Chilton WS, Benson DM (2002) Pyrrolnitrin production by Burkholderia cepacia and biocontrol of rhizoctonia stem rot of poinsettia. Biol Control 25:56–63. doi:10.1016/S1049-9644(02)00044-0
Kadir J, Rahman MA, Mahmud TMM, Abdul Rahman R, Begum MM (2008) Extraction of antifungal substances from Burkholderia cepacia with antibiotic activity against Colletotrichum gloeosporioides on papaya (Carica papaya). Int J Agri Biol 10:15–20
Kamaruzaman S, Dikin A (2005) Biochemical and physiological characterization of Burkholderia cepacia as biological control agent. Int J Agri Biol 3:385–388
Kang Y, Carlson R, Tharpe W, Schell MA (1998) Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Appl Environ Microbiol 64:3939–3947
Karabulut OA, Baykal N (2004) Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packing. Crop Prot 23:431–435
Katiyar V, Goel R (2004) Siderophore mediated plant growth promotion at low temperature by mutant of fluorescent pseudomonad. Plant Growth Regul 42:239–244. doi:10.1023/B:GROW.0000026477.10681.d2
Ker CK (2001) Sensitivity of mango anthracnose pathogen, Colletotrichum gloeosporioides, to the fungicide prochloraz in Taiwan. Proc Natl Sci Counc 25(3):174–179
Kulminskaya AA, Tomsen KK, Shabalin KA, Sidorenko IA, Eneyskaya EV, Savel’ev AN, Neustroev KN (2001) Isolation, enzymatic properties, and mode of action of an exo-1,3-β-glucanase from Trichoderma viride. Euro JBiochem 23:6123–6131
Leelavathy KM (1969) Effects of growth-regulating substances on fungi. Can J Microbiol 15:713–721. doi:10.1139/m69-126
Leonian LH, Lilly VG (1937) Is heteroauxin a growth promoting substance? Am J Bot 24:129–135
Lim Y, Shin SH, Lee SI, Kim IS, Rhee JH (1998) Iron repressibility of siderophore and transferrin-binding protein in Staphylococccus aureus. FEMS Microbiol Lett 163:19–24. doi:10.1016/S0378-1097(98)00143-8
Lipuma JJ (2003) Burkholderia cepacia complex as human pathogens. J Nematol 35:212–217
Loper JE, Henkels MD (1999) Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol 65:5357–5363
Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–445
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
O’Sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Okoh IA, Ajisebutu S, Babalola GO, Trejo-Hernandez MR (2001) A study of the potentials of a Burkholderia cepacia strain (RQ1) in the biodegradation of heavy crude oil (Maya). Int Microbiol 4:83–87
Páez M, Martínez-Nieto P, Bernal-Castillo J (2005) Siderophore producing Pseudomonas as pathogenic Rhizoctonia solani and Botrytis cinerea antagonists. Universitas Scientiarum 10:65–74
Parke JL, Gurian-Sherman D (2001) Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39:225–258
Rahman MA, Kadir J, Mahmud TMM, Abdul Rahman R, Begum MM (2007) Screening of antagonistic bacteria for biocontrol activities on Colletotrichum gloeosporioides in papaya. Asian J Plant Sci 6:12–20
Roitman JN, Mahoney NE, Janisiewicz WJ (1990) Production and composition of phenylpyrrole metabolites produced by Pseudomonas cepacia. Appl Microbiol Biotechnol 34:381–386
SAGARPA (2007) Servicio de Información Agroalimentaria y Pesquera. Recuperado el 11 de 06 de 2008, de http://www.siap.gob.mx/
Sandy M, Butler A (2009) Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109:4580–4595
Santoyo G, Valencia-Cantero E, Orozco-Mosqueda MC, Peña-Cabriales JJ, Farías-Rodríguez R (2010) Papel de los sideróforos en la actividad antagónica de pseudomonas fluorescens zum80 hacia hongos fitopatógenos. Terra Latinoamericana 28:53–60
Schmidt S, Blom JF, Pernthaler J, Berg G, Baldwin A, Mahenthiralingam E, Eberl L (2009) Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ Microbiol 11:1422–1437
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shenker M, Oliver I, Helmann M, Hadar Y, Chen Y (1992) Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J Plant Nutr 15:2173–2182
Silveira Mello AF, Zamboni Machado AC, Bedendo IP (2004) Development of Colletotrichum gloeosporioides isolated from green pepper in different culture media, temperatures, and light regimes. Sci Agric 61(5):542–544
Snow GA (1954) Mycobactin, a growth factor for Mycobacterium johnei: II. Degradation and identification of fragments. J Chem Soc 49:2588–2596
Sritharan M (2000) Iron as a candidate in virulence and pathogenesis in mycobacteria and other microorganisms. World J Microbiol Biotechnol 16:769–780
Stevens CV, Khan A, Lu JY, Wilson CL, Pusey PL, Igwegbe ECK, Kabwe K, Mafolo Y, Chalutz E, Droby S (1997) Integration of ultraviolet (UV-C) light with yeast treatment for control of postharvest storage rots of fruits and vegetables. Biol Control 10:98–103
Stoyanova M, Pavlina I, Moncheva P, Bogatzevska N (2007) Biodiversity and incidence of Burkholderia species. Biotechnol Biotechnol Equip 21:306–310
Suresh PV, Chandrasekaran M (1998) Utilization of prawn waste for chitinase production by the marine fungus Beauveria bassiana by solid state fermentation. World J Microbiol Biotechnol 14:655–660
Thahir Basha S, Suvarna J, Hemalatha TM, Eswara Reddy NP (2010) Compatibility of native potential bioagents with different fungicides against Colletotrichum gloeosporioides penz. causing mango anthracnose. Bioscan 5:19–20
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Wong DK, Gobin J, Horwitz MA, Gibson BW (1996) Characterization of exochelins of Mycobacterium avium: evidence for saturated and unsaturated acid for acid and ester forms. J Bacteriol 178:6394–6398
Xi K, Stephens JHG, Verma R (1996) Application of formulated rhizobacteria against root rot of field pea. Plant Pathol 45:1150–1158
Acknowledgments
We express our thanks to Dr. Eugene L. Madsen for his valuable help in the proper use of the English language. We extend our thanks to the Referees (anonymus) for suggestions which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de los Santos-Villalobos, S., Barrera-Galicia, G.C., Miranda-Salcedo, M.A. et al. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides . World J Microbiol Biotechnol 28, 2615–2623 (2012). https://doi.org/10.1007/s11274-012-1071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1071-9