Abstract
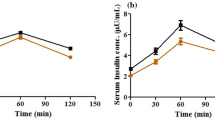
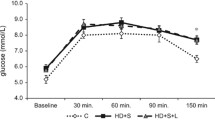
Metabolic syndrome (MetS) represents a cluster of related metabolic abnormalities, including central obesity, hypertension, dyslipidemia, hyperglycemia, and insulin resistance. These metabolic derangements present significant risk factors for chronic kidney disease that carries to loss of essential micronutrients, which accelerates comorbidity apparition. The work aimed was to evaluate the trace element homeostasis regarding morphological adaptations and renal function in MetS early-onset. Fifty male Wistar rats were divided into two groups: (a) control group and (b) hypercaloric diet group that developed MetS early-onset after 3 months. Classical zoometric parameters do not show changes; however, biochemical modifications were observed such as hyperglycemia, protein glycation, insulin resistance, dyslipidemia, hyperinsulinemia, and hypoadiponectinemia. MetS early-onset group observed renal structural modifications, but no functional changes. The structural modifications observed were minimal glomerular injury, glomerular basement membrane thickening, as well as mesangial and tubular cells that showed growth and proliferation. In serum and kidney (cortex and medulla), the concentrations of Zn, Fe, Cr, Mg, Mn, Cu, Co, and Ni were no differences between the experimental groups, but excretory fractions of these were lower in the hypercaloric diet group. In conclusion, MetS early-onset coexist renal structural modification and a hyperreabsorptive activity of essential trace elements that avoid its loss; thus, the excretory fraction of oligo-elements could be used a biomarker of early renal injury caused by metabolic diseases in the clinical practice.



Similar content being viewed by others
References
Alberti KGMM, Zimmet P, Shaw J (2005) The metabolic syndrome - a new worldwide definition. Lancet 366:1059–1062
Cleeman JI (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc 285:2486–2497. https://doi.org/10.1097/00019048-200106000-00021
Simmons RK, Alberti KGMM, Gale EAM, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich Mirchov I, Ben-Nakhi A, Reaven G, Hama Sambo B, Mendis S, Roglic G (2010) The metabolic syndrome: useful concept or clinical tool? Report of a WHO expert consultation. Diabetologia 53:600–605
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120:1640–1645
Rosyid FN (2017) Issue 10 Page 4206 International Journal of Research in Medical Sciences Rosyid FN. Int J Res Med Sci 5:4206–4213. https://doi.org/10.18203/2320-6012.ijrms20174548
Vaquero Alvarez M, Aparicio-Martinez P, Fonseca Pozo FJ, Valle Alonso J, Blancas Sánchez IM, Romero-Saldaña M (2020) A sustainable approach to the metabolic syndrome in children and its economic burden. Int J Environ Res Public Health 17:1891. https://doi.org/10.3390/ijerph17061891
Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie C, Carnethon M, Kaplan R, Giachello A, Gallo L, Loehr L, Avilés-Santa L (2014) Prevalence of metabolic syndrome among hispanics/latinos of diverse background: the Hispanic community health study/study of Latinos. Diabetes Care 37:2391–2399. https://doi.org/10.2337/dc13-2505
Nichols GA, Moler EJ (2011) Metabolic syndrome components are associated with future medical costs independent of cardiovascular hospitalization and incident diabetes. Metab Syndr Relat Disord 9:127–133. https://doi.org/10.1089/met.2010.0105
Curtis LH, Hammill BG, Bethel MA, Anstrom KJ, Gottdiener JS, Schulman KA (2007) Costs of the metabolic syndrome in elderly individuals: findings from the cardiovascular health study. Diabetes Care 30:2553–2558. https://doi.org/10.2337/dc07-0460
Ohashi Y, Thomas G, Nurko S, Stephany B, Fatica R, Chiesa A, Rule AD, Srinivas T, Schold JD, Navaneethan SD, Poggio ED (2013) Association of metabolic syndrome with kidney function and histology in living kidney donors. Am J Transplant 13:2342–2351. https://doi.org/10.1111/ajt.12369
Szczuko M, Kaczkan M, Drozd A, Maciejewska D, Palma J, Owczarzak A, Marczuk N, Rutkowski P, Małgorzewicz S (2019) Comparison of fatty acid profiles in a group of female patients with chronic kidney diseases (CKD) and metabolic syndrome (MetS)−similar trends of changes, Different Pathophysiology. Int J Mol Sci 20. https://doi.org/10.3390/ijms20071719
Litwin M, Niemirska A (2014) Metabolic syndrome in children with chronic kidney disease and after renal transplantation. Pediatr Nephrol 29:203–216
Thethi T, Kamiyama M, Kobori H (2012) The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep 14:160–169. https://doi.org/10.1007/s11906-012-0245-z
Lin JW, Chang YC, Li HY, Chien YF, Wu MY, Tsai RY, Hsieh YC, Chen YJ, Hwang JJ, Chuang LM (2009) Cross-sectional validation of diabetes risk scores for predicting diabetes, metabolic syndrome, and chronic kidney disease in Taiwanese. Diabetes Care 32:2294–2296. https://doi.org/10.2337/dc09-0694
Siddiqui K, Bawazeer N, Joy SS (2014) Variation in macro and trace elements in progression of type 2 diabetes. Sci World J 2014:1–9. https://doi.org/10.1155/2014/461591
Matsumura M, Nakashima A, Tofuku Y (2000) Electrolyte disorders following massive insulin overdose in a patient with type 2 diabetes. Intern Med 39:55–57. https://doi.org/10.2169/internalmedicine.39.55
Badran M, Morsy R, Soliman H, Elnimr T (2016) Assessment of trace elements levels in patients with type 2 diabetes using multivariate statistical analysis. J Trace Elem Med Biol 33:114–119. https://doi.org/10.1016/j.jtemb.2015.10.006
Barbagallo M, Dominguez LJ, Galioto A et al (2003) Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Asp Med 24:39–52
Samadi A, Isikhan SY, Tinkov AA, Lay I, Doşa MD, Skalny AV, Skalnaya MG, Chirumbolo S, Bjørklund G (2019) Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clin Nutr 39:1849–1856. https://doi.org/10.1016/j.clnu.2019.07.026
Sobczak AIS, Stefanowicz F, Pitt SJ, Ajjan RA, Stewart AJ (2019) Total plasma magnesium, zinc, copper and selenium concentrations in type-I and type-II diabetes. BioMetals 32:123–138. https://doi.org/10.1007/s10534-018-00167-z
Khan FA, Al Jameil N, Arjumand S et al (2015) Comparative study of serum copper, Iron, magnesium, and zinc in type 2 diabetes-associated proteinuria. Biol Trace Elem Res 168:321–329. https://doi.org/10.1007/s12011-015-0379-3
Tonelli M, Wiebe N, Hemmelgarn B et al (2009) Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med 7:25
Jankowska M, Rutkowski B, Dębska-Ślizień A (2017) Vitamins and microelement bioavailability in different stages of chronic kidney disease. Nutrients 9. https://doi.org/10.3390/nu9030282
Treviño S, Sánchez-Lara E, Sarmiento-Ortega VE, Sánchez-Lombardo I, Flores-Hernández JÁ, Pérez-Benítez A, Brambila-Colombres E, González-Vergara E (2015) Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3 [V10O28]·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J Inorg Biochem 147:85–92. https://doi.org/10.1016/j.jinorgbio.2015.04.002
Treviño S, Vázquez-Roque RA, López-López G, Perez-Cruz C, Moran C, Handal-Silva A, González-Vergara E, Flores G, Guevara J, Díaz A (2017) Metabolic syndrome causes recognition impairments and reduced hippocampal neuronal plasticity in rats. J Chem Neuroanat 82:65–75. https://doi.org/10.1016/j.jchemneu.2017.02.007
Diaz A, Escobedo C, Treviño S, Chávez R, Lopez-Lopez G, Moran C, Guevara J, Venegas B, Muñoz-Arenas G (2018) Metabolic syndrome exacerbates the recognition memory impairment and oxidative-inflammatory response in rats with an intrahippocampal injection of amyloid beta 1-42. Oxidative Med Cell Longev 2018:1358057–1358013. https://doi.org/10.1155/2018/1358057
Santamaria-Juarez C, Atonal-Flores F, Diaz A et al (2020) Aortic dysfunction by chronic cadmium exposure is linked to multiple metabolic risk factors that converge in anion superoxide production. Arch Physiol Biochem:1–9. https://doi.org/10.1080/13813455.2020.1726403
Treviño S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, Lopez-Lopez G, Muñoz-Arenas G, Morales-Medina JC, Toxqui V, Venegas B, Diaz A (2015) A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse 69:421–433. https://doi.org/10.1002/syn.21832
Sarmiento-Ortega V, Brambila E, Flores-Hernández J, Díaz A, Peña-Rosas U, Moroni-González D, Aburto-Luna V, Treviño S (2018) The NOAEL metformin dose is ineffective against metabolic disruption induced by chronic cadmium exposure in Wistar rats. Toxics 6:55. https://doi.org/10.3390/toxics6030055
Treviño S, Waalkes MP, Flores Hernández JA, León-Chavez BA, Aguilar-Alonso P, Brambila E (2015) Chronic cadmium exposure in rats produces pancreatic impairment and insulin resistance in multiple peripheral tissues. Arch Biochem Biophys 583:27–35. https://doi.org/10.1016/j.abb.2015.07.010
Farriol M, Rosselló J, Schwartz S (1997) Body surface area in Sprague-Dawley rats. J Anim Physiol Anim Nutr (Berl) 77:61–65. https://doi.org/10.1111/j.1439-0396.1997.tb00738.x
Raij L, Azar S, Keane W (1984) Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26:137–143. https://doi.org/10.1038/ki.1984.147
Nishiyama A, Yoshizumi M, Hitomi H et al (2004) The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol 15:306–315. https://doi.org/10.1097/01.asn.0000108523.02100.e0
Duarte FO, Sene-Fiorese M, Cheik NC, Maria ASLS, de Aquino AE Jr, Oishi JC, Rossi EA, Garcia de Oliveira Duarte AC, Dâmaso AR (2012) Food restriction and refeeding induces changes in lipid pathways and fat deposition in the adipose and hepatic tissues in rats with diet-induced obesity. Exp Physiol 97:882–894. https://doi.org/10.1113/expphysiol.2011.064121
Westerterp KR (2006) Perception, passive overfeeding and energy metabolism. Physiol Behav 89:62–65. https://doi.org/10.1016/j.physbeh.2005.12.014
Lejk A, Myśliwiec M, Myśliwiec A (2019) Effect of eating resistant starch on the development of overweight, obesity, and disorders of carbohydrate metabolism in children. Pediatr Endocrinol Diabetes Metab 25:81–84. https://doi.org/10.5114/pedm.2019.85818
Liao CC, Sheu WHH, Lin SY, Lee WJ, Lee IT (2020) The relationship between abdominal body composition and metabolic syndrome after a weight reduction program in adult men with obesity. Diabetes Metab Syndr Obes Targets Ther 13:1–8. https://doi.org/10.2147/DMSO.S228954
Qiu Y, Zhao Q, Gu Y, Wang N, Yu Y, Wang R, Zhang Y, Zhu M, Liu X, Jiang Y, Zhao G (2019) Association of metabolic syndrome and its components with decreased estimated glomerular filtration rate in adults. Ann Nutr Metab 75:168–178. https://doi.org/10.1159/000504356
Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD (2011) Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 6:2364–2373. https://doi.org/10.2215/CJN.02180311
Rashidbeygi E, Safabakhsh M, Delshadaghdam S et al (2019) Metabolic syndrome and its components are related to a higher risk for albuminuria and proteinuria: evidence from a meta-analysis on 10,603,067 subjects from 57 studies. Diabetes Metab Syndr Clin Res Rev 13:830–843
Chen J, Kong X, Jia X, Li W, Wang Z, Cui M, Xu D (2017) Association between metabolic syndrome and chronic kidney disease in a Chinese urban population. Clin Chim Acta 470:103–108. https://doi.org/10.1016/j.cca.2017.05.012
Lerman LO, Lerman A (2011) The metabolic syndrome and early kidney disease: another link in the chain? Rev Esp Cardiol 64:358–360. https://doi.org/10.1016/j.recesp.2011.01.005
El-Khashab SO, Gamil M, Ali AY et al (2019) Chemerin level and the relation to insulin resistance in chronic kidney disease. Saudi J Kidney Dis Transpl 30:1381–1388. https://doi.org/10.4103/1319-2442.275482
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Ren Physiol 311:F1087–F1108
Xu H, Carrero JJ (2017) Insulin resistance in chronic kidney disease. Nephrology 22:31–34
Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS (2007) A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Ren Physiol 292:292–F627. https://doi.org/10.1152/ajprenal.00278.2006
Kim Y, Park CW (2019) Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int J Mol Sci 20
Shen YY, Peake PW, Charlesworth JA (2008) Review article: adiponectin: its role in kidney disease. Nephrology 13:528–534
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295. https://doi.org/10.1038/nm788
Ouedraogo R, Wu X, Xu S-Q, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ (2006) Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes 55:1840–1846. https://doi.org/10.2337/db05-1174
Zhang Y, Yang S, Cui X, Yang J, Zheng M, Jia J, Han F, Yang X, Wang J, Guo Z, Chang B, Chang B (2019) Hyperinsulinemia can cause kidney disease in the IGT stage of OLETF rats via the INS/IRS-1/PI3-K/Akt signaling pathway. J Diabetes Res 2019:1–12. https://doi.org/10.1155/2019/4709715
Isshiki K, He Z, Maeno Y, Ma RC, Yasuda Y, Kuroki T, White GS, Patti ME, Weir GC, King GL (2008) Insulin regulates SOCS2 expression and the mitogenic effect of IGF-1 in mesangial cells. Kidney Int 74:1434–1443. https://doi.org/10.1038/ki.2008.403
Masson E, Wiernsperger N, Lagarde M, El Bawab S (2005) Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides. Biochem J 388:537–544. https://doi.org/10.1042/BJ20041506
Thrailkill KM, Clay Bunn R, Fowlkes JL (2009) Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine 35:1–10
Mariappan MM, De Silva K, Sorice GP et al (2014) Combined acute hyperglycemic and hyperinsulinemic clamp induced profibrotic and proinflammatory responses in the kidney. Am J Phys Cell Physiol 306:C202–C211. https://doi.org/10.1152/ajpcell.00144.2013
Higgins SP, Tang Y, Higgins CE, Mian B, Zhang W, Czekay RP, Samarakoon R, Conti DJ, Higgins PJ (2018) TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal 43:1–10
Kim S II, Choi ME (2012) TGF-β-activated kinase-1: new insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res Clin Pract 31:94–105
Morrisey K, Evans RA, Wakefield L, Phillips AO (2001) Translational regulation of renal proximal tubular epithelial cell transforming growth factor-β1 generation by insulin. Am J Pathol 159:1905–1915. https://doi.org/10.1016/S0002-9440(10)63037-4
Waheed F, Dan Q, Amoozadeh Y, Zhang Y, Tanimura S, Speight P, Kapus A, Szászi K (2013) Central role of the exchange factor GEF-H1 in TNF-α-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells. Mol Biol Cell 24:1068–1082. https://doi.org/10.1091/mbc.E12-09-0661
Vallon V (2011) The proximal tubule in the pathophysiology of the diabetic kidney. Am J Phys Regul Integr Comp Phys 300:R1009
Liu Y, Huang H, Gao R, Liu Y (2020) Dynamic phenotypes and molecular mechanisms to understand the pathogenesis of diabetic nephropathy in two widely used animal models of type 2 diabetes mellitus. Front Cell Dev Biol 8. https://doi.org/10.3389/fcell.2020.00172
Nunes S, Alves A, Preguiça I, Barbosa A, Vieira P, Mendes F, Martins D, Viana SD, Reis F (2020) Crescent-like lesions as an early signature of nephropathy in a rat model of prediabetes induced by a hypercaloric diet. Nutrients 12:12. https://doi.org/10.3390/nu12040881
Aghadavod E, Soleimani A, Amirani E, Gholriz Khatami P, Akasheh N, Sharafati Chaleshtori R, Shafabakhsh R, Banikazemi Z, Asemi Z (2020) Comparison between biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy and type 2 diabetes mellitus. Iran J Kidney Dis 14:31–35
Gill V, Kumar V, Singh K et al (2019) Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules:9
Savelieff MG, Callaghan BC, Feldman EL (2020) The emerging role of dyslipidemia in diabetic microvascular complications. Curr Opin Endocrinol Diabetes Obes 27:115–123
Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M (2005) Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54:2328–2335. https://doi.org/10.2337/diabetes.54.8.2328
Hirano T (2018) Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb 25:771–782
Chevalier RL (2016) The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Ren Physiol 311:F145–F161
Hallow KM, Gebremichael Y, Helmlinger G, Vallon V (2017) Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: a modeling analysis. Am J Physiol Ren Physiol 312:F819–F835. https://doi.org/10.1152/ajprenal.00497.2016
Gilbert RE (2017) Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66:791–800
Resnick LM, Barbagallo M, Gupta RK, Laragh JH (1993) Ionic basis of hypertension in diabetes mellitus. Role of hyperglycemia. Am J Hypertens 6:413–417. https://doi.org/10.1093/ajh/6.5.413
Aguilar MV, Saavedra P, Arrieta FJ, Mateos CJ, González MJ, Meseguer I, Martínez-Para MC (2007) Plasma mineral content in type-2 diabetic patients and their association with the metabolic syndrome. Ann Nutr Metab 51:402–406. https://doi.org/10.1159/000108108
Karganov MY, Alchinova IB, Tinkov AA, Medvedeva YS, Lebedeva MA, Ajsuvakova OP, Polyakova MV, Skalnaya MG, Burtseva TI, Notova SV, Khlebnikova NN, Skalny AV (2020) Streptozotocin (STZ)-induced diabetes affects tissue trace element content in rats in a dose-dependent manner. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02090-2
Tinkov AA, Gatiatulina ER, Popova EV, Polyakova VS, Skalnaya AA, Agletdinov EF, Nikonorov AA, Skalny AV (2017) Early high-fat feeding induces alteration of trace element content in tissues of juvenile male Wistar rats. Biol Trace Elem Res 175:367–374. https://doi.org/10.1007/s12011-016-0777-1
Farhadnejad H, Asghari G, Mirmiran P, Yuzbashian E, Azizi F (2016) Micronutrient intakes and incidence of chronic kidney disease in adults: Tehran lipid and glucose study. Nutrients 8:217. https://doi.org/10.3390/nu8040217
Martín-del-Campo F, Batis-Ruvalcaba C, González-Espinoza L et al (2012) Dietary micronutrient intake in peritoneal dialysis patients: relationship with nutrition and inflammation status. Perit Dial Int 32:183–191. https://doi.org/10.3747/pdi.2010.00245
Bossola M, Di Stasio E, Viola A et al (2014) Dietary intake of trace elements, minerals, and vitamins of patients on chronic hemodialysis. Int Urol Nephrol 46:809–815. https://doi.org/10.1007/s11255-014-0689-y
Tang X, Shay NF (2001) Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr 131:1414–1420. https://doi.org/10.1093/jn/131.5.1414
Eshed I, Elis A, Lishner M (2001) Plasma ferritin and type 2 diabetes mellitus: a critical review. Endocr Res 27:91–97. https://doi.org/10.1081/ERC-100107172
Haap M, Fritsche A, Mensing HJ, Hring HU, Stumvoll M (2003) Association of high serum ferritin concentration with glucose intolerance and insulin resistance in healthy people. Ann Intern Med 139:869–871
Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, Kandhro GA (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122:1–18. https://doi.org/10.1007/s12011-007-8062-y
Kaur B, Henry J (2014) Micronutrient status in type 2 diabetes: a review. Adv Food Nutr Res 71:55–100. https://doi.org/10.1016/B978-0-12-800270-4.00002-X
Wilson JG, Lindquist JH, Grambow SC, et al (2003) Potential role of increased iron stores in diabetes. In: American Journal of the Medical Sciences. Lippincott Williams and Wilkins, pp. 332–339
Mooren FC, Krüger K, Völker K, Golf SW, Wadepuhl M, Kraus A (2011) Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects - a double-blind, placebo-controlled, randomized trial. Diabetes. Obes Metab 13:281–284
Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, Rodríguez-Morán M (2004) Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab 30:253–258. https://doi.org/10.1016/S1262-3636(07)70116-7
Korc M (1983) Manganese action on pancreatic protein synthesis in normal and diabetic rats. Am J Physiol Gastrointest Liver Physiol 8:G628–G634. https://doi.org/10.1152/ajpgi.1983.245.5.g628
Juanola-Falgarona M, Cándido-Fernández J, Salas-Salvadó J, Martínez-González MA, Estruch R, Fiol M, Arija-Val V, Bulló M, for the PREDIMED Study Investigators (2013) Association between serum ferritin and osteocalcin as a potential mechanism explaining the iron-induced insulin resistance. PLoS One 8:8. https://doi.org/10.1371/journal.pone.0076433
Vallon V, Thomson SC (2012) Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74:351–375. https://doi.org/10.1146/annurev-physiol-020911-153333
Barbato A, Cappuccio FP, Folkerd EJ, Strazzullo P, Sampson B, Cook DG, Alberti KGMM (2004) Metabolic syndrome and renal sodium handling in three ethnic groups living in England. Diabetologia 47:40–46. https://doi.org/10.1007/s00125-003-1260-z
Cappuccio FP, Strazzullo P, Siani A, Trevisan M (1996) Increased proximal sodium reabsorption is associated with increased cardiovascular risk in men. J Hypertens 14:909–914. https://doi.org/10.1097/00004872-199607000-00015
Vallon V, Huang DY, Deng A et al (2002) Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13:1865–1871. https://doi.org/10.1097/01.ASN.0000016441.41118.57
Vallon V, Thomson SC (2020) The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 16:317–336
Makhlough A, Makhlough M, Shokrzadeh M, Mohammadian M, Sedighi O, Faghihan M (2015) Comparing the levels of trace elements in patients with diabetic nephropathy and healthy individuals. Nephrourol Mon 7:e28576. https://doi.org/10.5812/numonthly.28576
Al-Timimi DJ, Sulieman DM, Hussen KR (2014) Zinc status in type 2 diabetic patients: relation to the progression of diabetic nephropathy. J Clin Diagnostic Res 8:CC04
Dahan I, Thawho N, Farber E et al (2018) The Iron-Klotho-VDR Axis is a major determinant of proximal convoluted tubule injury in Haptoglobin 2-2 genotype diabetic nephropathy patients and mice. J Diabetes Res 2018. https://doi.org/10.1155/2018/7163652
Dominguez JH, Liu Y, Kelly KJ (2015) Renal iron overload in rats with diabetic nephropathy. Physiol Rep 3. https://doi.org/10.14814/phy2.12654
Lu Q, Zhai Y, Cheng Q, Liu Y, Gao X, Zhang T, Wei Y, Zhang F, Yin X (2013) The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp Physiol 98:934–945. https://doi.org/10.1113/expphysiol.2012.068361
Acknowledgments
Authors thank Dr. Francisco Ramos Collazo (Bioterio “Claude Bernard”, BUAP) for his assistance and the donation of the animals used in this work. We express our gratitude to Clinical Laboratory “Los Ángeles” by the facilities to realize the biochemical determinations. Thanks to Professor Thomas Edwards PhD., for editing the English language text.
Funding
The Vicerrectoria de Investigación y Posgrado [VIEP; TRMS-NAT19-1] through Ygnacio Martınez Laguna, CONACyT and the “Sistema Nacional de Investigadores” of Mexico provided financial support for this research project [CNSS, 000536].
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Solís, C.N., Hernández-Fragoso, H., Aburto-Luna, V. et al. Kidney Adaptations Prevent Loss of Trace Elements in Wistar Rats with Early Metabolic Syndrome. Biol Trace Elem Res 199, 1941–1953 (2021). https://doi.org/10.1007/s12011-020-02317-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02317-2