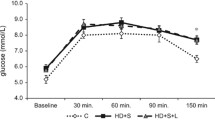
Abstract
Purpose
The study aimed to investigate the potential nephroprotective effects of vitamin D3 in metabolic syndrome (MetS) and the molecular basis of the underlying mechanisms of its action.
Methods
MetS was induced in adult male Wistar rats by adding fructose (10%) to every day drinking water and salt (3%) to the diet. Six weeks after fructose/salt consumption, fasting serum lipid profile and uric acid levels were determined, an oral glucose tolerance test (OGTT) was performed and kidney function was checked. MetS rats were then treated orally with vitamin D3 (10 µg/kg/day) for 6 weeks. At the end of the study period (12 weeks), the OGTT test was reperformed, anthropometrical parameters were measured, urine, blood and tissue samples were collected and the animals were euthanised.
Results
The incidence of MetS was confirmed 6 weeks after fructose/salt consumption, when the rats exhibited significant weight gain, dyslipidemia, hyperuricemia, insulin resistance, hyperinsulinemia and impaired glucose tolerance. After 12 weeks, MetS rats displayed markedly declined renal function alongside with extravagant renal histopathological damages and interstitial fibrosis. Furthermore, significantly enhanced renal oxidative stress and inflammation were manifested. Vitamin D3 supplementation in MetS rats significantly reversed all the above-mentioned deleterious effects.
Conclusion
The study has indeed provided mounting evidence of the promising therapeutic potential of vitamin D3 against development and progression of MetS-induced nephropathy. A new insight has been introduced into the crucial role of dipeptidyl peptidase-4 inhibition and sirtuin-1/5′adenosine monophosphate-activated protein kinase activation in the renoprotective effects of vitamin D3.










Similar content being viewed by others
Abbreviations
- AGEs:
-
Advanced glycation end products
- AMPK:
-
5′Adenosine monophosphate-activated protein kinase
- Ang II:
-
Angiotensin II
- ANOVA:
-
Analysis of variance
- ATIR:
-
Ang II type 1 receptor
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- BW:
-
Body weight
- CKD:
-
Chronic kidney disease
- 1,25(OH)2D3:
-
1,25-Dihydroxy vitamin D3
- DPP-4:
-
Dipeptidyl peptidase-4
- ELISA:
-
Enzyme-linked immunosorbent assay
- FSG:
-
Fasting serum glucose
- FSI:
-
Fasting serum insulin
- GLP-1:
-
Glucagon-like peptide-1
- H&E:
-
Hematoxylin and eosin
- HbA1c:
-
Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis Model Assessment of Insulin Resistance
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MAPK:
-
Mitogen-activated protein kinase
- MDA:
-
Malondialdehyde
- MetS:
-
Metabolic syndrome
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- OGTT:
-
Oral glucose tolerance test
- P/T:
-
Phosphorylated/total
- RAAS:
-
Renin angiotensin aldosterone system
- ROS:
-
Reactive oxygen species
- S.E.M:
-
Standard error of the mean
- SIRT1:
-
Sirtuin-1
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TGF-β1:
-
Transforming growth factor-β1
- TNF-α:
-
Tumor necrosis factor-α
- UACR:
-
Urinary albumin/creatinine ratio
- VDR:
-
Vitamin D receptor
- WC:
-
Waist circumference
References
Srikanthan K, Feyh A, Visweshwar H et al (2016) Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci 13:25–38. https://doi.org/10.7150/ijms.13800
Wortsman J, Matsuoka LY, Chen TC et al (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693. https://doi.org/10.1093/ajcn/72.3.690
Romacho T, Elsen M, Rohrborn D et al (2014) Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 210:733–753. https://doi.org/10.1111/apha.12246
Ouchi N, Parker JL, Lugus JJ et al (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85. https://doi.org/10.1038/nri2921
Rüster C, Wolf G (2013) The role of the renin–angiotensin–aldosterone system in obesity-related renal diseases. Semin Nephrol 33:44–53. https://doi.org/10.1016/j.semnephrol.2012.12.002
Han T, Meng X, Shan R et al (2018) Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int J Obes 42:1336. https://doi.org/10.1038/s41366-018-0074-5
Zuo L, Ushio-Fukai M, Ikeda S et al (2005) Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II Type 1 receptor stimulation in vascular smooth muscle cells. Arterioscler Thromb Vasc Bio 25:1824–1830. https://doi.org/10.1161/01.ATV.0000175295.09607.18
Sachse A, Wolf G (2007) Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18:2439–2446. https://doi.org/10.1681/ASN.2007020149
Ferder M, Inserra F, Manucha W et al (2013) The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin–angiotensin system. Am J Physiol Cell Physiol 304:C1027–C1039. https://doi.org/10.1152/ajpcell.00403.2011
McMullan CJ, Borgi L, Curhan GC et al (2017) The effect of vitamin D on renin–angiotensin system activation and blood pressure: a randomized control trial. J Hypertens 35:822–829. https://doi.org/10.1097/HJH.0000000000001220
Vaidya A, Williams JS (2012) The relationship between vitamin D and the renin–angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metab Clin Exp 61:450–458. https://doi.org/10.1016/j.metabol.2011.09.007
Li Y (2011) Chapter 40—vitamin d and the renin–angiotensin system. Vitamin D, 3rd edn. Academic Press, San Diego, pp 707–723
Aroor A, Zuberek M, Duta C et al (2016) Angiotensin II stimulation of DPP4 activity regulates megalin in the proximal tubules. Int J Mol Sci 17:780. https://doi.org/10.3390/ijms17050780
Liu J, Li X, Lu Q et al (2019) AMPK: a balancer of the renin–angiotensin system. Biosci Rep 39:BSR20181994. https://doi.org/10.1042/BSR20181994
Nguyen LT, Chen H, Pollock C et al (2017) SIRT1 reduction is associated with sex-specific dysregulation of renal lipid metabolism and stress responses in offspring by maternal high-fat diet. Sci Rep 7:8982. https://doi.org/10.1038/s41598-017-08694-4
Abdallah HM, El-Bassossy HM, Mohamed GA et al (2016) Phenolics from Garciniamangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement Altern Med 16:359. https://doi.org/10.1186/s12906-016-1340-5
Divi S, Bellamkonda R, Dasireddy SK (2012) Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose fed insulin resistant and STZ induced diabetic wistar rats: a comparative study. Asian J Pharm Clin Res 5:67–72
Esteghamati A, Ashraf H, Khalilzadeh O et al (2010) Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond) 7:26. https://doi.org/10.1186/1743-7075-7-26
Den Alaa El A, Hussien ETNI, Allam MM et al (2018) The potential effect of vitamin D on rats with fatty liver induced by a choline-deficient diet. Benha Med J 35:67. https://doi.org/10.4103/bmfj.bmfj_3_17
Prietl B, Treiber G, Pieber TR et al (2013) Vitamin D and immune function. Nutrients 5:2502–2521. https://doi.org/10.3390/nu5072502
Shin J-W, Seol I-C, Son C-G (2010) Interpretation of animal dose and human equivalent dose for drug development. 대한한의학회지 31:1–7
Novelli E, Diniz Y, Galhardi C et al (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41:111–119. https://doi.org/10.1258/002367707779399518
Fish R, Danneman PJ, Brown M et al (2011) Anesthesia and analgesia in laboratory animals. Academic Press, San Diego, pp 240–282
Meiattini F, Prencipe L, Bardelli F et al (1978) The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. Clin Chem 24:2161–2165
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Grove TH (1979) Effect of reagent pH on determination of high-density lipoprotein cholesterol by precipitation with sodium phosphotungstate–magnesium. Clin Chem 25:560–564
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Henry RJ, Cannon DC, Winkelman JW (1974) Clinical chemistry: principles and technics
Comitti R, Racchetti G, Gnocchi P et al (1987) A monoclonal-based, two-site enzyme immunoassay of human insulin. J Immunol Methods 99:25–37. https://doi.org/10.1016/0022-1759(87)90028-7
Fossati P, Prencipe L, Berti G (1980) Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Kaplan A, Glucose KA (1984) Clin Chem. The CV Mosby Co, St Louis, p 436
Fawcett J, Scott J (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159. https://doi.org/10.1136/jcp.13.2.156
Chen X, Chen Y, Shen Z (2004) A competitive ELISA for albumin in rat urine. J Immunoassay Immunochem 25:81–89. https://doi.org/10.1081/IAS-120027228
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Pollak N, Dölle C, Ziegler M (2007) The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem J 402:205–218. https://doi.org/10.1042/BJ20061638
Kiernan JA (1999) Histological and histochemical methods: theory and practice. Shock 12:479
Drury R, Wallington E (1980) General staining procedures. Carleton's histological techniques. Oxford University Press, USA, pp 125–150
Alegret M, Roglans N, Laguna J (2011) Fructose consumption and leptin resistance: what have we learnt from animal studies. Leptin: hormonal functions, dysfunctions and clinical uses. Nova Science Publishers Inc, Hauppauge, pp 210–230
Johnson RJ, Nakagawa T, Sanchez-Lozada LG et al (2013) Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62:3307–3315. https://doi.org/10.2337/db12-1814
Eren OC, Ortiz A, Afsar B et al (2019) Multilayered interplay between fructose and salt in development of hypertension: what has been revealed so far. J Hypertens 73:265–272. https://doi.org/10.1161/HYPERTENSIONAHA.118.12150
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol 4:37. https://doi.org/10.3389/fendo.2013.00037
Veronique D, Yves S, Jacklyn L et al (2012) Excessive fructose intake causes 1, 25-(OH) 2D3-dependent inhibition of intestinal and renal calcium transport in growing rats. Am J Physiol Endocrinol Metab 12:1303–1313. https://doi.org/10.1152/ajpendo.00582.2012
Li YC, Qiao G, Uskokovic M et al (2004) Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89:387–392. https://doi.org/10.1016/j.jsbmb.2004.03.004
Siragy HM, Carey RM (2010) Role of the intrarenal renin–angiotensin–aldosterone system in chronic kidney disease. Am J Nephrol 31:541–550. https://doi.org/10.1159/000313363
du Toit E.F. and D.G. Donner (2012) Myocardial insulin resistance: an overview of its causes, effects, and potential therapy. Insulin resistance 189.
Zhang X, Lerman LO (2017) The metabolic syndrome and chronic kidney disease. Transl Res 183:14–25. https://doi.org/10.1016/j.trsl.2016.12.004
Higashijima Y, Tanaka T, Yamaguchi J et al (2015) Anti-inflammatory role of DPP-4 inhibitors in a nondiabetic model of glomerular injury. Am J Physiol Renal Physiol 308:F878–F887. https://doi.org/10.1152/ajprenal.00590.2014
Farah LX, Valentini V, Pessoa TD et al (2015) The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am J Physiol Renal Physiol 310:F123–F127. https://doi.org/10.1152/ajprenal.00394.2015
Yang P, Feng J, Peng Q et al (2019) Advanced glycation end products: potential mechanism and therapeutic target in cardiovascular complications under diabetes. Oxid Med Cell Longev 2019:9570616. https://doi.org/10.1155/2019/9570616
Dong YJ, Liu N, Xiao Z et al (2014) Renal protective effect of sirtuin 1. J Diabetes Res 2014:843786. https://doi.org/10.1155/2014/843786
Okabe K, Yaku K, Tobe K et al (2019) Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci 26:34. https://doi.org/10.1186/s12929-019-0527-8
Chen I-C, Kuo C-S, Wu C-C et al (2018) Chronic hyperuricemia impairs blood flow recovery in the ischemic hindlimb through suppression of endothelial progenitor cells. Oncotarget 9:9285. https://doi.org/10.18632/oncotarget.24290
Szrejder M, Piwkowska A (2019) AMPK signalling: implications for podocyte biology in diabetic nephropathy. Biol Cell 111:109–120. https://doi.org/10.1111/boc.201800077
Kim M-J, Park I-J, Yun H et al (2010) AMP-activated protein kinase antagonizes pro-apoptotic extracellular signal-regulated kinase activation by inducing dual-specificity protein phosphatases in response to glucose deprivation in HCT116 carcinoma. J Biol Chem 285:14617–14627. https://doi.org/10.1074/jbc.M109.085456
Wang D, Warner GM, Yin P et al (2013) Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am J Physiol Renal Physiol 304:F938–F947. https://doi.org/10.1152/ajprenal.00706.2012
Yang S, Li A, Wang J et al (2018) Vitamin D receptor: a novel therapeutic target for kidney diseases. Curr Med Chem 25:3256–3271. https://doi.org/10.2174/0929867325666180214122352
Agarwal R, Hynson JE, Hecht TJ et al (2011) Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80:1073–1079. https://doi.org/10.1038/ki.2011.207
López-Jaramillo P, Gómez-Arbeláez D, López-López J et al (2014) The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig 18:37–45. https://doi.org/10.1515/hmbci-2013-0053
Vaidya A, Forman JP, Hopkins PN et al (2011) 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angio Aldo S 12:311–319. https://doi.org/10.1177/1470320310391922
Lubkowska A, Radecka A, Bryczkowska I et al (2015) Serum adiponectin and leptin concentrations in relation to body fat distribution, hematological indices and lipid profile in humans. Int J Environ Res Public Health 12:11528–11548. https://doi.org/10.3390/ijerph120911528
Al-Shoumer KA, Al-Essa TM (2015) Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J Diabetes 6:1057. https://doi.org/10.4239/wjd.v6.i8.1057
Toriu N, Yamanouchi M, Hiramatsu R et al (2018) Preservation of renal function by intensive glycemic control. Endocrinol Diabetes Metab Case Rep. https://doi.org/10.1530/EDM-17-0136
Pörsti IH (2008) Expanding targets of vitamin D receptor activation: downregulation of several RAS components in the kidney. Kidney Int 74:1371–1373. https://doi.org/10.1038/ki.2008.424
Canale D, de Braganca AC, Goncalves JG et al (2014) Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: role of oxidative stress and renin-angiotensin system. PLoS ONE 9:e103055. https://doi.org/10.1371/journal.pone.0103055
Esaki H, Tachi T, Goto C et al (2017) Renoprotective effect of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Front Pharmacol 8:835. https://doi.org/10.3389/fphar.2017.00835
Enciso PL, Wang L, Kawahara Y et al (2015) Dietary vitamin D3 improves postprandial hyperglycemia in aged mice. Biochem Biophys Res Commun 461:165–171. https://doi.org/10.1016/j.bbrc.2015.04.008
Chang E, Kim Y (2016) Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. J Nutr 32:702–708. https://doi.org/10.1016/j.nut.2015.12.032
Chang E, Kim Y (2017) Vitamin D insufficiency exacerbates adipose tissue macrophage infiltration and decreases AMPK/SIRT1 activity in obese rats. Nutrients 9:338. https://doi.org/10.3390/nu9040338
Zhang Y, Leung DY, Richers BN et al (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188:2127–2135. https://doi.org/10.4049/jimmunol.1102412
Haddad Kashani H, Seyed Hosseini E, Nikzad H et al (2018) The effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic hemodialysis: a randomized, double-blind, placebo-controlled trial. Front Pharmacol 9:50. https://doi.org/10.3389/fphar.2018.00050
Potthoff SA, Stamer S, Grave K et al (2016) Chronic p38 mitogen-activated protein kinase inhibition improves vascular function and remodeling in angiotensin II-dependent hypertension. J Renin-Angio-Aldo S 17:1470320316653284. https://doi.org/10.1177/1470320316653284
Xu Z, Li W, Han J et al (2017) Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2). Sci Rep 7:44911. https://doi.org/10.1038/srep44911
Acknowledgements
No financial support was received. The authors acknowledge Prof. Dr. Dina Sabry, Professor of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, for her valuable help in performing the molecular biology analysis.
Author information
Authors and Affiliations
Contributions
All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest and have disclosed no financial or personal relationship with organizations that could potentially be perceived as influencing the described research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
We confirm that this study has been approved by the Ethical Committee for Animal Handling at Zagazig University and has therefore been performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wahba, N.S., Ghareib, S.A., Abdel-Ghany, R.H. et al. Renoprotective effects of vitamin D3 supplementation in a rat model of metabolic syndrome. Eur J Nutr 60, 299–316 (2021). https://doi.org/10.1007/s00394-020-02249-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02249-6