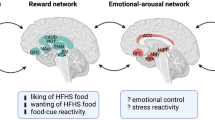
Abstract
Purpose of Review
There is compelling evidence in the clinical population that long-term weight loss secondary to bariatric surgery is mitigated by the reemergence of maladaptive feeding behaviors and in some cases new onset substance abuse.
Recent Findings
A review of the current literature suggests that physical restructuring of the GI tract during WLS alters secretion of feeding peptides and nutrient-sensing mechanisms that directly target the brain’s endogenous reward system, the mesolimbic dopamine system.
Summary
Post-surgical changes in GI physiology augment activation of the mesolimbic system. In some patients, this process may contribute to a reduced appetite for palatable food whereas in others it may support maladaptive motivated behavior for food and chemical drugs. It is concluded that future studies are required to detail the timing and duration of surgical-induced changes in GI-mesolimbic communication to more fully understand this phenomenon.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Adult Obesity Facts | Overweight & Obesity | CDC. (2019, January 31). Retrieved May 29, 2019, from https://www.cdc.gov/obesity/data/adult.html
Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7. https://doi.org/10.1001/jama.2012.39.
Arterburn DE, Maciejewski ML, Tsevat J. Impact of morbid obesity on medical expenditures in adults. Int J Obes. 2005;29(3):334–9. https://doi.org/10.1038/sj.ijo.0802896.
Drenick EJ, Bale GS, Seltzer F, Johnson DG. Excessive mortality and causes of death in morbidly obese men. JAMA. 1980;243(5):443–5. https://doi.org/10.1001/jama.1980.03300310031018.
Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1275–91. https://doi.org/10.1152/ajpregu.00185.2014.
Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the US. Int J Obes (2005). 2013;37(6):889–91. https://doi.org/10.1038/ijo.2012.159.
Wang Y, Song Y, Chen J, Zhao R, Xia L, Cui Y, et al. Roux-en-Y gastric bypass versus sleeve gastrectomy for super super obese and super obese: systematic review and meta-analysis of weight results, comorbidity resolution. Obes Surg. 2019;29(6):1954–64. https://doi.org/10.1007/s11695-019-03817-4.
Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev. 2015;95(1):47–82. https://doi.org/10.1152/physrev.00007.2014.
Casazza K, Brown A, Astrup A, Bertz F, Baum C, Brown MB, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr. 2015;55(14):2014–53. https://doi.org/10.1080/10408398.2014.922044.
Cooksey-Stowers K, Schwartz MB, Brownell KD. Food swamps predict obesity rates better than food deserts in the United States. Int J Environ Res Public Health. 2017;14(11). https://doi.org/10.3390/ijerph14111366.
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. https://doi.org/10.1016/S2213-8587(16)30107-3.
Hruby A, Hu FB. The epidemiology of obesity: A big picture. PharmacoEconomics. 2015;33(7):673–89. https://doi.org/10.1007/s40273-014-0243-x.
Kim TJ, von dem Knesebeck O. Income and obesity: what is the direction of the relationship? A systematic review and meta-analysis. BMJ Open. 2018;8(1). https://doi.org/10.1136/bmjopen-2017-019862.
Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metabolism. 2015;4(6):437–60. https://doi.org/10.1016/j.molmet.2015.03.005.
Bliss ES, Whiteside E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol. 2018;9. https://doi.org/10.3389/fphys.2018.00900.
de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301(1):E187–95. https://doi.org/10.1152/ajpendo.00056.2011.
Sanmiguel C, Gupta A, Mayer EA. Gut microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep. 2015;4(2):250–61. https://doi.org/10.1007/s13679-015-0152-0.
Kang JH, Le QA. Effectiveness of bariatric surgical procedures. Medicine. 2017;96(46):e8632. https://doi.org/10.1097/MD.0000000000008632.
Geary N, Bächler T, Whiting L, Lutz TA, Asarian L. RYGB progressively increases avidity for a low-energy, artificially sweetened diet in female rats. Appetite. 2016;98:133–41. https://doi.org/10.1016/j.appet.2015.11.029.
Mathes CM, Letourneau C, Blonde GD, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats progressively decreases the proportion of fat calories selected from a palatable cafeteria diet. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R952–9. https://doi.org/10.1152/ajpregu.00444.2015.
Mumphrey MB, Hao Z, Townsend RL, Patterson LM, Münzberg H, Morrison CD, et al. Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit. Int J Obes (2005). 2016;40(6):921–8. https://doi.org/10.1038/ijo.2016.38.
Washington MC, Mhalhal TR, Johnson-Rouse T, Berger J, Heath J, Seeley R, et al. Roux-en-Y gastric bypass augments the feeding responses evoked by gastrin-releasing peptides. J Surg Res. 2016;206(2):517–24. https://doi.org/10.1016/j.jss.2016.08.057.
•• Sirohi S, Richardson BD, Lugo JM, Rossi DJ, Davis JF. Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity. 2017;25(7):1228–36. https://doi.org/10.1002/oby.21839. Discovered that GHSR-1a signaling is altered in mesolimbic dopamine neurons in rats behaviorally characterized for increased alcohol intake and reduced hedonic food intake.
Orellana ER, Jamis C, Horvath N, Hajnal A. Effect of vertical sleeve gastrectomy on alcohol consumption and preferences in dietary obese rats and mice: a plausible role for altered ghrelin signaling. Brain Res Bull. 2018;138:26–36. https://doi.org/10.1016/j.brainresbull.2017.08.004.
Biegler JM, Freet CS, Horvath N, Rogers AM, Hajnal A. Increased intravenous morphine self-administration following Roux-en-Y gastric bypass in dietary obese rats. Brain Res Bull. 2016;123:47–52. https://doi.org/10.1016/j.brainresbull.2015.08.003.
Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S89–96. https://doi.org/10.1210/jc.2008-1641.
Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33(4):595–622. https://doi.org/10.1210/er.2011-1044.
Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118(11):1844–55. https://doi.org/10.1161/CIRCRESAHA.116.307591.
Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring, Md.). 2014;22(5):E13–20. https://doi.org/10.1002/oby.20649.
Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18(6):648–51. https://doi.org/10.1007/s11695-007-9265-1.
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23(11):1922–33. https://doi.org/10.1007/s11695-013-1070-4.
Still CD, Wood GC, Chu X, Manney C, Strodel W, Petrick A, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring, Md.). 2014;22(3):888–94. https://doi.org/10.1002/oby.20529.
Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en-Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg. 2013;23(1):50–5. https://doi.org/10.1007/s11695-012-0754-5.
Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628–36; discussion 636–637. https://doi.org/10.1097/SLA.0b013e3182a5034b.
DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis Off J Am Soc Bariatric Surg. 2010;6(4):347–55. https://doi.org/10.1016/j.soard.2009.11.015.
Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry. 2012;72:354–60. https://doi.org/10.1016/j.biopsych.2012.01.035.
Davis JF, Tracy AL, Schurdak JD, Magrisso IJ, Grayson BE, Seeley RJ, et al. Roux en Y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23(7):920–30. https://doi.org/10.1007/s11695-013-0884-4.
Dutta S, Morton J, Shepard E, Peebles R, Farrales-Nguyen S, Hammer LD, et al. Methamphetamine use following bariatric surgery in an adolescent. Obes Surg. 2006;16(6):780–2. https://doi.org/10.1381/096089206777346646.
Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg Obes Relat Dis. 2008;4(5):647–50. https://doi.org/10.1016/j.soard.2008.01.004.
Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, et al. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One. 2012;7(11):e49121. https://doi.org/10.1371/journal.pone.0049121.
Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, et al. Gastric bypass increases ethanol and water consumption in diet-induced obese rats. Obes Surg. 2012;22(12):1884–92. https://doi.org/10.1007/s11695-012-0749-2.
Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–75. https://doi.org/10.1002/pds.1833.
Sirohi S, Skripnikova E, Davis JF. Vertical sleeve gastrectomy attenuates hedonic feeding without impacting alcohol drinking in rats. Obesity (Silver Spring, Md.). 2019;27(4):603–11. https://doi.org/10.1002/oby.22415.
King WC, Chen J-Y, Mitchell JE, Kalarchian M, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–25. https://doi.org/10.1001/jama.2012.6147.
Himes SM, Grothe KB, Clark MM, Swain JM, Collazo-Clavell ML, Sarr MG. Stop regain: a pilot psychological intervention for bariatric patients experiencing weight regain. Obes Surg. 2015;25(5):922–7. https://doi.org/10.1007/s11695-015-1611-0.
Tamboli RA, Breitman I, Marks-Shulman PA, Jabbour K, Melvin W, Williams B, et al. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity. 2014;22(7):1617–22. https://doi.org/10.1002/oby.2077.
Hao Z, Münzberg H, Rezai-Zadeh K, Keenan M, Coulon D, Lu H, et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes. 2015;39(5):798–805. https://doi.org/10.1038/ijo.2014.189.
Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiy S, et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Phys Regul Integr Comp Phys. 2007;293(4):R1474–89. https://doi.org/10.1152/ajpregu.00171.2007.
Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–52.
Marqués-Iturria I, Scholtens LH, Garolera M, Pueyo R, García-García I, González-Tartiere P, et al. Affected connectivity organization of the reward system structure in obesity. Neuroimage. 2015;111:100–6.
Tuominen L, Tuulari J, Karlsson H, Hirvonen J, Helin S, Salminen P, et al. Aberrant mesolimbic dopamine-opiate interaction in obesity. Neuroimage. 2015;122:80–6.
Geha P, Cecchi G, Todd Constable R, Abdallah C, Small DM. Reorganization of brain connectivity in obesity. Hum Brain Mapp. 2017;38:1403–20.
Avery JA, Powell JN, Breslin FJ, Lepping RJ, Martin LE, Patrician TM, et al. Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J Psychopharmacol (Oxford). 2017;31:1475–84.
Ho M-C, Chen VC-H, Chao S-H, Fang C-T, Liu Y-C, Weng J-C. Neural correlates of executive functions in patients with obesity. PeerJ. 2018;6:e5002.
Chen VC-H, Liu Y-C, Chao S-H, McIntyre RS, Cha DS, Lee Y, et al. Brain structural networks and connectomes: the brain-obesity interface and its impact on mental health. Neuropsychiatr Dis Treat. 2018;14:3199–208.
Karlsson HK, Tuulari JJ, Tuominen L, Hirvonen J, Honka H, Parkkola R, et al. Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol Psychiatry. 2016;21:1057–62.
Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, Cooney RN, et al. Roux-en-Y gastric bypass alters brain activity in regions that underlie reward and taste perception. PLoS One. 2015;10:e0125570.
Wiemerslage L, Zhou W, Olivo G, Stark J, Hogenkamp PS, Larsson EM, et al. A resting-state fMRI study of obese females between pre- and postprandial states before and after bariatric surgery. Eur J Neurosci. 2017;45:333–41.
Olivo G, Zhou W, Sundbom M, Zhukovsky C, Hogenkamp P, Nikontovic L, et al. Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: a longitudinal study. Sci Rep. 2017;7:6616.
Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes. 2018;42:785–93.
Pearce AL, Mackey E, Cherry JBC, Olson A, You X, Magge SN, et al. Effect of adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity (Silver Spring). 2017;25:1852–60.
Li P, Shan H, Liang S, et al. Sleeve gastrectomy recovering disordered brain function in subjects with obesity: a longitudinal fMRI study. Obes Surg. 2018;28:2421–8.
Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, et al. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 2016;23:103–12.
Zhang Y, Ji G, Xu M, Cai W, Zhu Q, Qian L, et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes. 2016;40:1558–65.
Liu L, Ji G, Li G, et al. Structural changes in brain regions involved in executive-control and self-referential processing after sleeve gastrectomy in obese patients. Brain Imaging Behav 2018.
Faulconbridge LF, Ruparel K, Loughead J, Allison KC, Hesson LA, Fabricatore AN, et al. Changes in neural responsivity to highly palatable foods following Roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: an fMRI study. Obesity (Silver Spring). 2016;24:1054–60.
Zoon HFA, de Bruijn SEM, Jager G, Smeets PAM, de Graaf C, Janssen IMC, et al. Altered neural inhibition responses to food cues after Roux-en-Y gastric bypass. Biol Psychol. 2018;137:34–41.
Zoon HFA, de Bruijn SEM, Smeets PAM, de Graaf C, Janssen IMC, Schijns W, et al. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav Brain Res. 2018;353:194–202.
Karlsson HK, Tuulari JJ, Hirvonen J, Lepomäki V, Parkkola R, Hiltunen J, et al. Obesity is associated with white matter atrophy: a combined diffusion tensor imaging and voxel-based morphometric study. Obesity. 2013;21:2530–7.
Bohon C, Geliebter A. Change in brain volume and cortical thickness after behavioral and surgical weight loss intervention. Neuroimage Clin. 2019;21:101640.
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24.
Volkow ND, Wang G-J, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18.
Hankir MK, Seyfried F, Hintschich CA, Diep TA, Kleberg K, Kranz M, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25:335–44.
Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond Ser B Biol Sci. 2006;361:1149–58.
Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. JNeurosci. 2004;24:1050–7.
Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, et al. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci U S A. 2016;113:2520–5.
Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–9.
Cook JB, Hendrickson LM, Garwood GM, Toungate KM, Nania CV, Morikawa H. Junk food diet-induced obesity increases D2 receptor autoinhibition in the ventral tegmental area and reduces ethanol drinking. PLoS One. 2017;12:e0183685.
Koyama S, Mori M, Kanamaru S, Sazawa T, Miyazaki A, Terai H, et al. Obesity attenuates D2 autoreceptor-mediated inhibition of putative ventral tegmental area dopaminergic neurons. Physiol Rep. 2014;2:e12004.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41.
de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1:37.
Wu C, Garamszegi SP, Xie X, Mash DC. Altered dopamine synaptic markers in postmortem brain of obese subjects. Front Hum Neurosci. 2017;11:386.
Pak K, Kim S-J, Kim IJ. Obesity and brain positron emission tomography. Nucl Med Mol Imaging. 2018;52:16–23.
van der Zwaal EM, de Weijer BA, van de Giessen EM, Janssen I, Berends FJ, van de Laar A, et al. Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss. Eur Neuropsychopharmacol. 2016;26:1190–200.
Blum K, Thanos PK, Wang G-J, Febo M, Demetrovics Z, Modestino EJ, et al. The food and drug addiction epidemic: targeting dopamine homeostasis. Curr Pharm Des. 2018;23:6050–61.
de Weijer BA, van de Giessen E, Janssen I, Berends FJ, van de Laar A, Ackermans MT, et al. Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia. 2014;57:1078–80.
Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20:369–74.
•• Hamilton J, Swenson S, Hajnal A, Thanos PK. Roux-en-Y gastric bypass surgery normalizes dopamine D1, D2, and DAT levels. Synapse. 2018;72:e22058. Found restoration of D2-receptor binding in striatum and hence mesolimbic dopamine function in RYGB rats relative to obese controls.
Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. Mastery in bariatric surgery: the long-term surgeon learning curve of Roux-en-Y gastric bypass. Ann Surg. 2018;267:489–94.
Merrer JL, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412.
Hyytia P. Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;45:697–701.
Bazov I, Kononenko O, Watanabe H, et al. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. AddictBiol. 2011.
Nogueiras R, Romero-Picó A, Vazquez MJ, Novelle MG, López M, Diéguez C. The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts. 2012;5:196–207.
Joutsa J, Karlsson HK, Majuri J, et al. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res Neuroimaging. 2018;276:41–5.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Sirohi S, Skripnikova E, Davis JF. Vertical sleeve gastrectomy attenuates hedonic feeding without impacting alcohol drinking in rats. Obesity (Silver Spring). 2019;27:603–11.
Hankir MK, Patt M, Patt JTW, Becker GA, Rullmann M, Kranz M, et al. Suppressed fat appetite after Roux-en-Y gastric bypass surgery associates with reduced brain μ-opioid receptor availability in diet-induced obese male rats. Front Neurosci. 2017;10.
Pasternak GW, Pan Y-X. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–317.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5. https://doi.org/10.1097/SLA.0b013e3180caa3e3.
Ivezaj V, Stoeckel LE, Avena NM, Benoit SC, Conason A, Davis JF, et al. Obesity and addiction: can a complication of surgery help us understand the connection? Obes Rev. 2017;18:765–75. https://doi.org/10.1111/obr.12542.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. Retrieved from. https://doi.org/10.1016/j.cmet.2006.01.004\n.
Dar MS, Chapman WH, Pender JR, Drake AJ, O’Brien K, Tanenberg RJ, et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg. 2012;22(7):1077–83. https://doi.org/10.1007/s11695-012-0624-1.
Chambers AP, Jessen L, Ryan KK, Sisley S, Wilsonpérez HE, Stefater MA, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–8. https://doi.org/10.1053/j.gastro.2011.05.050.
Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–58. https://doi.org/10.1210/en.2011-1443.
Fortin SM, Roitman MF. Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav. 2017;176:17–25. https://doi.org/10.1016/j.physbeh.2017.03.019.
Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2016;41(7):1917–28. https://doi.org/10.1038/npp.2015.362.
Cummings DE, Weigle DS, Frayo RS, Breen P, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30. https://doi.org/10.1056/NEJMoa012908.
Camiña JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145(2):930–40. https://doi.org/10.1210/en.2003-0974.
Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S Am. 2009;106:11318–23. https://doi.org/10.1073/pnas.0812809106.
Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Investig. 2006;116(12):3229–39. https://doi.org/10.1172/JCI29867.
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–72. https://doi.org/10.1172/JCI26002.
Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25:113–7. https://doi.org/10.1016/j.tips.2004.01.010.
Petersen PS, Woldbye DPD, Madsen AN, Egerod KL, Jin C, Lang M, et al. In vivo characterization of high basal signaling from the ghrelin receptor. Endocrinology. 2009;150(11):4920–30. https://doi.org/10.1210/en.2008-1638.
Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, … Piomelli D. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953). Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12955147.
Fu J, Gaetani S, Oveisi F, Verme JL, Serrano A, Rodríguez De Fonseca F, et al. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953):90–3. https://doi.org/10.1038/nature01921.
Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limón P, Ren X, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341(6147):800–2. https://doi.org/10.1126/science.1239275.
•• Hankir MK, Seyfried F, Hintschich CA, Diep TA, Kleberg K, Kranz M, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metabol. 2017;25(2):335–44. https://doi.org/10.1016/j.cmet.2016.12.006. Discovered that OEA-PPAR-α signaling increase mesolimbic dopamine secretion.
Bottin JH, Thomas EL, Balogun B, Bech PR, Ghatei MA, Moorthy K, et al. Changes in appetite, food intake, and appetite regulating hormones during acute weight loss induced by Roux-en-y gastric bypass and low-calorie diet. Obes Facts. 2015;8:66–272. https://doi.org/10.1159/000382140.
Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes. 2011;35(5):642–51. https://doi.org/10.1038/ijo.2010.174.
Habegger KM, Heppner KM, Amburgy SE, Ottaway N, Holland J, Raver C, et al. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2014;63(2):505–13. https://doi.org/10.2337/db13-0511.
Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci. 2016;9:66–70.
Menzies JRW, Skibicka KP, Leng G, Dickson SL. Ghrelin, reward and motivation. Endocr Dev. 2013;25:101–11.
Abizaid A, Liu Z-W, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39.
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37.
Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–23.
Barkholt P, Pedersen PJ, Hay-Schmidt A, Jelsing J, Hansen HH, Vrang N. Alterations in hypothalamic gene expression following Roux-en-Y gastric bypass. Mol Metab. 2016;5:296–304.
Blum K, Bailey J, Gonzalez AM, et al. Neuro-genetics of reward deficiency syndrome (RDS) as the root cause of “addiction transfer”: a new phenomenon common after bariatric surgery. J Genet Syndr Gene Ther. 2011;2012.
Backman O, Stockeld D, Rasmussen F, Näslund E, Marsk R. Alcohol and substance abuse, depression and suicide attempts after Roux-en-Y gastric bypass surgery. Br J Surg. 2016;103:1336–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Julianna N. Brutman, Sunil Sirohi, and Jon F. Davis each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Eating Disorders.
Rights and permissions
About this article
Cite this article
Brutman, J.N., Sirohi, S. & Davis, J.F. Recent Advances in the Neurobiology of Altered Motivation Following Bariatric Surgery. Curr Psychiatry Rep 21, 117 (2019). https://doi.org/10.1007/s11920-019-1084-2
Published:
DOI: https://doi.org/10.1007/s11920-019-1084-2