Abstract
Protocols were developed for the generation of haploid and doubled haploid plants from isolated microspores of carrot (Daucus carota L.). Forty-seven carrot accessions, including six inbred lines, 11 cultivars, 20 F1s, two BC1F1s, four F2s, one F3, and three F4s, were screened to evaluate the genotype influence on isolated microspore embryogenesis over 4 years. Twenty-eight accessions responded by producing embryos and/or calli. A cytological analysis showed that two modes of carrot microspore embryogenesis exist: an indirect route via calli (C mode), and a direct route via embryos (E mode). Eleven accessions were in the C mode, and 17 were in both modes. The highest production rates were in 10Y25 (a European Nantes cultivar) with 27 calli and 307 embryos, and 100Q6 (a semi-Nantes F1 hybrid) with 176 calli and 114 embryos. The time period to produce embryos or calli differed significantly between 2 and 6 months. Cold and heat pretreatment generally had a negative impact on the induction of microspore embryogenesis, but a short pretreatment showed a positive influence on some accessions. Twenty-eight lines regenerated plants from the primary individual embryos or calli of three accessions were established to analyze the ploidy level. The percentage of spontaneous diploidization showed very wide differences among the accessions and lines. Differences in leaf color intensity, leaf size, and leaf dissection were found among haploid, doubled haploid, and triploid plants.
Similar content being viewed by others
Introduction
Carrot (Daucus carota L.) is an out-crossing biennial species, and one of the most economically important vegetable crops worldwide (Peterson and Simon 1986). Its flowering is initiated after a vernalization period of eight to 10 weeks and the crop is typically bred in an annual cycle (Bradeen and Simon 2007). Selection and production of inbred lines is time consuming and difficult to achieve. Even with 6 years of self-pollination by conventional breeding methods, only about 98 % homozygosity could be achieved (Germanà 2006; Ferrie and Möllers 2010). Moreover, the vigor of early-generation carrot inbred lines selected from open-pollinated cultivars decreases dramatically due to inbreeding depression (Peterson and Simon 1986).
A doubled haploid (DH) line is valuable for crop breeding programs because traits are fixed without multiple self-pollinating generations. It was first envisioned as a technique to accelerate the breeding process and to use for practical and basic research in many crops (Dunwell 2010; Ferrie and Caswell 2010; Germanà 2011). While efficient protocols for microspore embryogenesis induction have been developed to obtain haploid and DH plants in several crops (Maluszynski et al. 2003; Dunwell 2010; Ferrie and Caswell 2010), limited progress has been reported using anther cultures (Dohya et al. 1997; Tyukavin et al. 1999; Adamus and Michalik 2003; Górecka et al. 2005; Smýkalová et al. 2009;), isolated microspore cultures (IMC; Dohya et al. 1997; Górecka et al. 2010; Ferrie et al. 2011), or unfertilized ovule cultures (Kiełkowska and Adamus 2010) of Apiaceae species.
These species are considered to be recalcitrant to DH technologies, although carrot is a model plant for tissue culture and regeneration protocols are available for several species (Ferrie et al. 2011; Grzebelus et al. 2012). Practical utilization of the DH technique is hindered by the low efficiency of embryogenesis in carrot anther cultures. Only 0.8 % of 20,400 carrot anthers generated callus (Andersen et al. 1990), the highest efficiency accession was HCM A.C. about 5.6 embryos per 100 anthers among five cultivars (Górecka et al. 2005), and only six accessions demonstrated successful microspore embryogenesis among 39 different genotype donors (Zhuang et al. 2010). Moreover, carrot anthers are so small that they are difficult to manipulate, making the avoidance of interfering sporophytic anther wall tissues difficult when establishing calli or embryos.
Compared with anther culture, the procedure of IMC is simpler and more efficient. The growth course of microspores can be observed directly and interference with somatic tissue is avoided. The conditions leading to the induction and development of microspore-derived embryos vary depending on the species. Many factors influence microspore embryogenesis, including genotype, stage of microspore development, donor plant growth conditions, media composition, and culture conditions (Maluszynski et al. 2003; Dunwell 2010; Ferrie and Caswell 2010; Ahmadi et al. 2012). Matsubara et al. (1995) firstly obtained small calli from a few carrot-isolated microspores. Górecka et al. (2010) observed 42 androgenetic carrot plants that were all diploids out of 90 plantlets. Ferrie et al. (2011) obtained 17 regenerated lines that produced seeds for field evaluation. Given the limited success, the system of carrot IMC should be improved. The objective of this study was to evaluate the influence of two main factors, genotype and stress pretreatment, on carrot microspore embryogenesis based on the previous anther culture (Zhuang et al. 2010). Haploid, DH, and triploid plantlets were successfully obtained and two development modes of DH from microspores were observed.
Materials and methods
Plant material
From 2008 to 2011, 47 accessions were selected for genotype evaluation, including six inbred lines, 11 cultivars, 20 F1s, two BC1F1s, four F2s, one F3, and three F4s (Table 1). Daucus carota L. var. sativus (hereafter referred to as var. s.) USDA inbreds HCM A.C. (an Imperator line), BETA III (an Imperator line), 2327 (a Nantes line), 3080B (an Imperator line), 6366B (an Imperator line), and 7262B (a dark purple outer phloem with orange core root line) were supplied by Dr. Philipp W. Simon, University of Wisconsin, USA. The white root wild accession D. c. PI 421301 was supplied by the North Central Regional Plant Introduction Station (NCRPIS), USA. Var. s. ‘Shitian’ (a Kuroda cultivar), ‘Gailiang Heitian’ (a Kuroda cultivar), ‘Finger’ (a small orange root Nantes cultivar), ‘Shanxi Jiangzi’ (a purple skin with yellow flesh root landrace), ‘Liangtouqi’ (an orange phloem with small yellow core root landrace), ‘Danvers’ (a North America primary cultivar), ‘Amsterdam’ (a European primary cultivar), ‘Nantes’ (a European primary cultivar), ‘Nantes 5’ (a European primary cultivar), ‘Hииox 336’ (a Nantes cultivar), ‘Touchon’ (a Nantes cultivar), ‘M1645’ (a Kuroda cultivar), ‘East Hongxiu’ (a Kuroda cultivar), and the white root wild accession D. c. ‘Songzi’ were supplied by the National Mid-term Genebank of Vegetable Genetic Resources, Chinese Academy of Agricultural Sciences. Var. s. ‘Hongguan’ (a semi-Nantes cultivar), ‘Kuroda’ (an Asian primary cultivar), ‘Hn1061’ (a Kuroda cultivar), Hn001 (a Kuroda line), Hn006 (a Kuroda line), and ‘Hn86’ (a Kuroda cultivar) were supplied by Beijing Honor Seed Co., Ltd. Var. s. ‘Nanco’ (a European Nantes cultivar), ‘Bolero’ (a European Nantes cultivar) and ‘Tino’ (a European Nantes cultivar) were introduced from Vilmorin Seed Co., Ltd. The donors of F1 and BC1F1 were hybrids arising from a cross or a back-cross between the accessions (detailed in Table 1), and F2, F3, and F4 were offspring from hybrids by self pollination for one, two, and three generations, respectively. The donors of 70198, 70Q68, 70Q75, 70Q78, 90W12, 90W30, 900C2, 90129, 90210, and 90278 were repeated for 2 years (Table 2).
Seeds of accessions were sown at the Changping Station of Chinese Academy of Agricultural Sciences in late July. Roots were harvested in early November and stored at 2–4 °C for about 4 months. Ten to 15 vernalized roots of each accession were selected and planted in plastic tunnels in mid-March where flowering was initiated. The vigor of the microspore is a crucial point for the success of IMC. So during the plant growth, any form of stress such as pesticide treatment, or dehydration was avoided. A minimal weekly preventive pesticide treatment was applied.
Microspore isolation and culture
In late April, plants gradually bolted and flowered. For each accession, 10–15 umbellets corresponding to late uninucleate or early binucleate stages were collected in the morning, which were determined by the bud size (mostly 0.8–1.4 mm in length) and morphology (flat inflorescence) of the flowers in the field, and were then evaluated using a Zeiss Axio 40 microscope (Zhuang et al. 2010). Collected buds were put into a tea basket, surface-sterilized with 75 % ethanol for 30 s, and 10 % (w/v) sodium hypochlorite for 15 min prior to rinsing them three times with sterilized distilled water. They were then gently crushed with a pestle in a mortar with 1 ml B5 (Gamborg et al. 1968) liquid medium with 13 % sucrose (pH = 5.8) added. The slurry was blended with B5 medium and then filtered through a 50 μm nylon mesh into a 10 ml centrifuge tube. The filtrate was centrifuged at 1,000 rpm for 5 min. The supernatant was discarded and 8 ml fresh B5 medium was added to resuspend the microspore sediment and centrifuged again. This procedure was repeated twice. After the last centrifugation the supernatant was decanted and the pellet was resuspended in NLN (Lichter 1982) liquid medium with 0.1 mg/l 2, 4-D and 0.1 mg/l NAA, 13 % sucrose (pH = 5.8) (Zhuang et al. 2010). The microspore density was adjusted to 1.5 × 105–2.5 × 105 microspores per ml NLN. The microspore suspension was dispensed at 3 ml per plate (60 mm × 15 mm Petri dish) with 50 μl of 1 % activated carbon using a handheld pipette. The Petri dishes were sealed with double layers of parafilm. All cultures were treated with a 33 °C heat shock for 2 days, and then incubated in darkness at 25 °C. The number of Petri dishes with embryos and/or calli was counted to calculate the ratios.
Cold and heat pretreatments
The benefits of various starvation and stress techniques have been reported in several plant species (Gu et al. 2004; Zoriniants et al. 2005; Dunwell 2010; Zhuang et al. 2010; Bhowmik et al. 2011; Chen et al. 2011; Ferrie et al. 2011; Seguí-Simarro et al. 2011; Takahira et al. 2011; Winarto and Teixeira da Silva 2011). A 28-day treatment of spikes at 4 °C was shown to lead to a much increased yield of microspore calli in cereals (Dunwell 2010). Cold (4 °C) and heat (32 °C) pretreatment had a positive impact on some accessions to produce embryos and calli in a carrot anther culture (Zhuang et al. 2010). In our experiment, eight accessions of 70Q75, 90210, 90225, 90251, 90278, 90285, 900C2, and 900Q1 with some embryos or calli growth in most cases were used in heat and cold pretreatment experiments (Table 1). The preparations of plant material and microspore isolation were the same as described above. Dishes containing isolated microspores were treated at treatment A (33 °C for 2 days without cold treatment), B (4 °C for 1 day, followed by 33 °C for 2 days), C (4 °C for 2 days, followed by 33 °C for 2 days), or D (4 °C for 3 days, followed by 33 °C for 2 days), and then incubated in darkness at 25 °C with two repetitions for each accession. The ratio of Petri dishes with embryos and/or calli was analyzed with ANOVA using SPSS 16.0 software.
Plantlet regeneration and acclimatization
Once embryos had grown into the cotyledon stage or calli had grown to a size of 1–2 mm in the NLN liquid medium, they were transferred to MS (Murashige and Skoog 1962) solid medium with 30 g/l sucrose, 6.5 g/l agar (pH = 5.8), and were cultured at 25 °C and a 16 h photoperiod. Some embryos and calli differentiated into secondary calli and/or embryos which were transferred to fresh media every 4 weeks. The plantlets with healthy roots were transplanted into jiffy pots with vermiculite, and were grown in a small shaded shed to gradually adapt them to the greenhouse conditions (temperature about 25–30/10–15 °C).
Ploidy identification and morphological characters of regenerated plants
In our experiments, the adapted plants from 70198, 70Q78, and 80Q54 were used to analyze spontaneous chromosome doubling. The ploidy level of young leaves was determined by a flow cytometer (FACSCalibur, BD, USA; Górecka et al. 2010; Ferrie et al. 2011). Approximately 2 cm2 leaf tissue was chopped several times with a razor blade in the presence of 2 ml of lysis buffer (15 mM Tris, 2 mM Na2EDTA, 0.5 mM spermine, 80 mM KCl, 20 mM NaCl, 0.1 % v/v TRITON X-100), and incubated for 1 min. The suspension was filtered through a 30-μm nylon filter to 5-ml centrifuge tube and centrifuged at 800 rpm for 10 min. The supernatant was discarded and 200 μl staining solution (75 μM propidium iodide, excited with a 488 nm laser) was added to the sediment. The samples were incubated in darkness at 4 °C for 20 min prior to analysis.
Flower morphology and fertility of early bolting plants were investigated. Pollen viability was evaluated according to the Alexander’s procedure (1969) based on differential staining of aborted (colored as light) and non-aborted grains (colored as magenta-red) using a Zeiss Axio 40 microscope. When the stigma was receptive, the flowers were self-pollinated with a small flannel brush twice everyday. Agronomic characters measured included leaf color intensity, leaf size, leaf dissection, and petiole color of haploid, DH and triploid plantlets.
Results
Modes of microspores embryogenesis
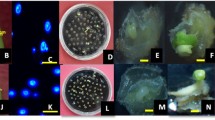
Górecka et al. (2010) noticed the first divisions of carrot immature microspores under a microscope about 2 weeks after culture initiation, while these structures could be observed with the naked eye after 3 weeks to 6 months. In our study, small embryos or calli were visible with the naked eye about 5 weeks after the IMC was established (Fig. 1a). According to the development of microspores from diverse accessions (Table 2), two main modes of embryogenesis could be distinguished. One type was an indirect route via calli (Fig. 1b–f, called C mode): the microspores firstly swelled into ellipses or spheres (Fig. 1b), split and were connected loosely with each other (Fig. 1c), then developed into calli (Fig. 1d). The color variation of the calli was found from a given donor plant in some accessions (Fig. 1e). The calli could differentiate into secondary embryos or adventitious shoots when they were transferred to the fresh MS solid medium (Fig. 1f). The other type of development observed was a direct route towards embryos (Fig. 1g–q, called E mode) where the microspores expanded to a tube-shaped structure about four times longer than the original size (about 15 μm), then developed into embryos like the zygote. Some pre-embryos would continue to differentiate into several secondary pre-embryos (Fig. 1i), then form polyembryos (Fig. 1j). Single, twin and poly-cotyledonary embryos were visible with the naked eye (Fig. 1o, p). Both primary and secondary embryos could directly grow into plantlets similar to seedlings (Fig. 1q). In total, there were 11 accessions in the C mode (inbred 900C2, cultivars 90210, 10334, and 10Y19, F1s 80Q52, 100Q1, and 10Q10, BC1F1 10E32, F2 10Q38, F4 90278, and F3 90285), 17 accessions (inbreds 70198 and 90W30, cultivars 90129, 90225, 10241, 10276, 10Y25, and 10Y30, F1s 70Q75, 70Q78, 80Q54, 90E12, 100Q3, and 100Q6, BC1F1 100Q9, F2 900Q1, and F4 90251) in both C and E modes, and none in E mode alone (Table 2).
Cytology of embryos and calli derived from carrot IMC. a Small embryos or calli visible with the naked eye. b Microspores ellipsoid or spherical shaped. c Multicellular aggregations and small callus formed. d Growing calli. e Color variation of calli transferred from a given donor plant, a yellow, b white. f Calli could differentiate into embryos, roots or shoots. g Microspores expanded longer four times than the primary length and divided into pre-embryos. h Growing microspores and a pre-embryo. i Embryo differentiated into several pre-embryos. j Globular polyembryos. k–n Single embryo at globular, torpedo and early cotyledonary stage. o Single cotyledonary embryo. p Cotyledonary polyembryos. q Regenerated plants developing from embryos. Nu nuclear, Ms microspore, Ca callus, PE pre-embryo, Em embryo. Bars = 50 μm. (Color figure online)
Influence of genotype
In this study 47 accessions (Table 1), three inbred lines, nine cultivars, nine F1s, two BC1F1s, two F2s, one F3, and two F4s formed calli and/or embryos (Table 2). The environmental conditions under which the donor plants grow can markedly influence culture responses (Dunwell 2010). Only one out of seven accessions responded in 2008, four out of 13 in 2009, 10 out of 14 in 2010, and 17 out of 23 in 2011 (Table 2). The production of calli and/or embryos varied greatly for different genotype accessions. The highest production rate of calli was found in 10276, 100Q6, and 100Q9 with 78, 176, and 78 calli, respectively. The highest production rate of embryos was 70198, 10Y25, and 100Q6 with 95, 307, and 114 embryos, respectively.
The time required for callus or embryo formation varied significantly (Table 2). Some accessions required about 4–6 months to form visible calli or embryos, such as 70Q78 with 192 days in 2008, 80Q52 with 182 days, 90278 with 130 days, 100Q1 with 111 days, 10Q10 with 108 days, and 10Q38 with 102 days. Generally, the production rates of these accessions were low and the vigor of calli or embryos was feeble. Most of them turned brown or gray after transferred to the MS solid medium. Others took 2–3 months to form calli or embryos, such as 80Q54 with 92 days, 10276 with 73 days, 10Y25 with 61 days, 100Q3 with 62 days, 100Q6 with 73 days, and 100Q9 with 78 days. The shortest time span was required by 900Q1 with only 38 days. This variation in rate of development was similar to findings by Górecka et al. (2010), and much longer than those observed in Brassica species (Fang et al. 2006; Zhang et al. 2008; Bhowmik et al. 2011; Ferrie and Bethune 2011; Takahira et al. 2011).
The ten different genotype accessions were selected for testing over different years (Table 2). Four accessions of 70Q78, 90W30, 900C2, and 90129 responded in both years, although the production rates of embryos and/or calli, the ratio of Petri dishes, and the time periods were different. The periods of 70Q78 were long with 192 days in 2008 and 177 days in 2009, respectively, while periods of 90W30, 900C2 and 90129 were short with 63, 69, 51 days in 2010 and 98, 94, and 88 days in 2011, respectively. Four accessions of 70198, 70Q75, 90210, and 90278 responded in a second year, and two accessions of 70Q68 and 90W12 showed no response.
Influence of cold and heat pretreatment
Stress factors acting directly on the microspores can act as a trigger for embryogenic induction in some crops but there were no significant differences in induction ratios of eight carrot accessions, although average treatment ratios were significant (Fig. 2). The ratio of treatment A was the highest with 11.9 %, while treatment D had the lowest ratio at 0.83 %, to indicate a negative impact of long cold treatment on most accessions. With the exception of 90278, seven other accessions responded to treatment A, with the highest ratio in 900Q1 at 35.3 %. There were only three, two and one accessions responding to treatments B, C, and D, respectively. However, cold pretreatment was positive for embryogenic induction in some accessions. The ratio of 90210 was 25.0 % at treatment B and 11.1 % at treatment A. The ratio of 900C2 was 35.2 % at treatment C but only 5.9 % at treatment A. Accession 90278 could be activated at treatment B while there was no response at treatment A (Fig. 2). This was similar to findings in carrot anther culture by Zhuang et al. (2010).
Effect of cold (4 °C) and heat (33 °C) pretreatment on carrot IMC. A 33 °C for 2 days without cold treatment; B 4 °C for 1 day, followed by 33 °C for 2 days; C 4 °C for 2 days, followed by 33 °C for 2 days; D 4 °C for 3 days, followed by 33 °C for 2 days. Average treatment ratios were calculated according to those of eight accessions and analyzed with ANOVA. a/ab/b were significant at the 0.05 level of probability
Plant regeneration and ploidy determination
After being transferred, some embryos grew into plantlets with three to four leaves in 1 month, some embryos and calli became brown or gray, stopped growth, and then died, and other embryos and calli continued to differentiate. There were 129 survivors among 289 embryos or calli transferred from 70198, 70Q78, and 80Q54 (Table 3). In accession 70198, only 37 out of 96 transferred embryos survived, and produced 143 plantlets, but no plantlet regenerated from the five surviving calli. In 70Q78, only 16 calli survived among 42 transferred, and produced 20 plantlets. 80Q54 had a high survival ratio of embryos or calli with 58.6 and 55.2 %, and produced 22 and 23 plantlets, respectively. One hundred and nine, eight and 37 plantlets from 70198, 70Q78, and 80Q54 successfully adapted to the greenhouse conditions, respectively.
With colchicine treatment in carrot IMC, DH plantlets could be obtained (Górecka et al. 2010; Ferrie et al. 2011), but there is little information about spontaneous chromosome doubling. Thirteen lines from individual primary embryos of 70198, three lines from individual calli of 70Q78, and 12 lines from seven embryos and five calli of 80Q54 were established to analyze the ploidy levels of plantlets (Table 4). The large lines was No. 8E of 70198 with 31 plantlets. Spontaneous diploidization could exist in both C and E modes of IMC, while the percentages were significantly different among the three accessions (Table 4). In accession 70198, 81.4 % were haploids, 17.5 % diploids, and 1.0 % triploids. In 70Q78, 57.1 % were haploids and 42.9 % diploids. In 80Q54, 33.3 % were haploids, 63.6 % diploids, and 3.0 % triploids.
Different ploidy plantlets were found in the same line (Table 4). The percentages of haploid and diploid were 94.4 % (17) and 5.6 % (1) for No. 3E line from 70198. The percentages of haploid and diploid were 66.7 % (8) and 33.3 % (4) for No. 4E line from 70198. The percentages of haploid, diploid and triploid were 83.9 % (26), 12.9 % (4), and 3.2 % (1) for No. 8E from 70198, respectively. The percentages of haploid, diploid and triploid were 14.3 % (1), 71.4 % (5), and 14.3 % (1) for No. 7E from 80Q54, respectively. This also showed the percentages of spontaneous diploidization were great differences among the different lines even from the same accession.
Morphological characters of regenerated plants
Several plantlets showed early bolting without any cold treatment even in culture or soon after transplanting (Fig. 3a, b). Some of them were fertile with normal anthers and many viable pollen grains (Fig. 3d). After self-pollination a few seeds were produced (Fig. 3c). Some were sterile with brown anthers (Fig. 3e) and few viable pollen grains (Fig. 3f). Several albino plantlets were found in 70198 (Fig. 3g). There were significant differences about the leaf color intensity, leaf size, and leaf dissection between the haploid, DH and triploid plants (Fig. 3h, i). The leaf length of haploids (Fig. 3hi, ii) was smaller than that of DH and triploids (Fig. 3hii, iii, iii). Figure 3h shows that the leaf color intensity of haploids and DH from 70198 was lighter than that of triploids, and that leaf dissection of haploids was higher than that of DH and triploids. Figure 3i shows that leaf dissection of haploids was slighter than that of DH from 70198, and that leaf color intensity of haploids was similar to DH. Petioles coloration was dark purple in some DH from 80Q54 (Fig. 3ji), but purple pigmentation was absent in other DH (Fig. 3jii).
Morphological characters of the plantlets derived from carrot IMC. a, b Some plantlets bolted without vernalization, arrow showed the umbellets. c Plantlets flower and set seed after self-pollination. d Anther with many viable (magenta-red in colour picture) pollen grains after Alexander staining. e Some plantlets were sterile with brown anthers. f Brown anther with few viable pollen grains. g Albino plantlet whose leaves were sensitive to the strong sunlight. h Comparison of plant leaf color intensity and phylliform from 70198, i haploid plant with green color and thin dissected leaves, ii diploid plant with light green color and large dissected leaves, iii triploid plant with deep green color and large dissected leaves. i Comparison of plant leaf size and phylliform from 70198, i haploid plant with short leaves, ii diploid plant with normal leaves. j Comparison of coloration in plant petiole from 80Q54, i diploid plant with green petiole, ii diploid plant with dark purple petiole. (Color figure online)
Discussion
Utilization of heterosis has been the main focus in carrot breeding since the discovery of genic-cytoplasmic male sterility (CMS; Peterson and Simon 1986; Bradeen and Simon 2007), but a lack of homozygous parents and a long breeding period highlight the potential for deriving hybrids. With DH production systems established, homozygous lines can be achieved in one generation, which would allow to reduce the time and cost of cultivar development (Dunwell 2010; Ferrie and Caswell 2010; Germanà 2011). The system has been effectively used in barley, wheat (Jauhar et al. 2009; Ferrie and Caswell 2010), maize (Smith et al. 2008; Chang and Coe 2009), and various Brassica species (Belmonte et al. 2006; Fang et al. 2006; Zhang et al. 2008; Ferrie and Bethune 2011; Takahira et al. 2011; Winarto and Teixeira da Silva 2011). The genotype is one of the crucial factors evaluated in many plants (Ferrie et al. 1995; Dunwell 2010). In previous studies, only several carrot cultivars and inbred lines were used, and little information was generated using IMC (Matsubara et al. 1995; Górecka et al. 2010; Ferrie et al. 2011). Based on a previous study (Zhuang et al. 2010), 47 carrot accessions were screened to evaluate the genotype effect on the IMC (Table 1). There were three inbred lines, nine cultivars, nine F1s, two BC1F1s, two F2s, one F3, and two F4s responding (Table 2). The most productive accessions were 70198 (an Imperator inbred line) with 32 calli and 95 embryos, 80Q54 (an Imperator F1 hybrid) with 67 calli and 56 embryos, 10276 (an Asian primary cultivar) with 78 calli and 19 embryos, 10Y25 (a European primary cultivar) with 27 calli and 307 embryos, 100Q6 (a semi-Nantes F1 hybrid) with 176 calli and 114 embryos, and 100Q9 (a semi-Nantes BC1F1 hybrid) with 78 calli and five embryos. This is the first report evaluating such a wide range of genotypes with such a high production of calli and embryos in a carrot IMC.
Irrespective of early events in the division of microspores, they can take a direct or an indirect mode to develop into an embryo (Ferrie and Caswell 2010). In Brassica species, microspores become swollen and start to divide after 2–3 days culture, and embryos can usually be detected about 10 days after culture initiation (Belmonte et al. 2006; Fang et al. 2006; Ferrie and Bethune 2011; Zhang et al. 2008), wherein the embryos develop directly and proceed through the globular, heart-shaped, torpedo, and cotyledonary stages. The indirect route involves a number of irregular and asynchronous divisions, which results in a callus, and it undergoes organogenesis to form embryos. In our study, two modes C and E were observed (Fig. 1, Table 2). Cytological analysis showed that initiation of microspores was significantly different between C and E mode: microspores that swelled like an ellipse or sphere would develop into calli (Fig. 1b, c, e), while those expanding into longer, tube-like shapes would form embryos (Fig. 1g, h). The period of callus or embryo formation in carrot was much longer, which coincided with results by Górecka et al. (2010). Some accessions required 4–6 months to form visible calli or embryos such as 70Q78 (a semi-Danvers F1 hybrid) after 192 days in 2008, or 80Q52 (a semi- Imperator F1 hybrid) after 182 days (Table 2). However, these calli or embryos were very weak, changed color to brown or gray, and stopped growth even after being transferred into fresh medium. Other accessions required 2–3 months to produce calli and/or embryos, which could continue to differentiate and grow into mature plantlets. For carrot IMC, the rapid initiation of microspore division would be one of the most important steps.
Prior to its incubation under normal conditions, yield of embryos or calli has been substantially improved by pretreatment of anthers or microspores in maize, wheat, barley, rice, and Brassica species. In potatoes, a pretreatment of 2 days at 6 °C is recommended (Wenzel et al. 1983). In cereals, a recommended 28-day treatment of the spikes at 4 °C leads to an increased microspore calli yield (Dunwell 2010). In carrot anther cultures, Andersen et al. (1990) found that no pretreatment or treatment of 1–2 days at 7 °C was the method of choice, and Zhuang et al. (2010) suggested that pretreatment at 4 °C for 2–3 days and then 32 °C for 2 days was positive in some accessions. This study showed that cold and heat pretreatment had a generally negative impact on the induction of microspore embryogenesis (Fig. 2). However, short cold and heat pretreatment was positive for some accessions, such as 90210 (a Kuroda cultivar) and 90278 (a dark purple outer phloem with orange core) at 4 °C for 1 day followed by 33 °C for 2 days, and 900C2 (a Kuroda inbred line) at 4 °C for 2 days followed by 33 °C for 2 days, thus confirming findings by Zhuang et al. (2010). This phenomenon has also been observed in some Brassica species (Gu et al. 2004).
When large numbers of haploid and infertile plants survive, the production efficiency of DH plants is reduced in providing materials useful for breeding programs. For some species, there is a high percentage of spontaneous chromosome doubling, which results in homozygous DH plants. If spontaneous doubling does not occur, or occurs at a low frequency, the haploid plants need to be treated with a doubling agent, such as colchicine, oryzalin, and trifluralin (Maluszynski et al. 2003; Castillo et al. 2009; Germanà 2011; Mohammadi et al. 2012). Cytogenetic analysis has shown that there were high rate of spontaneous diploidization in carrot anther culture (Zhuang et al. 2010). All androgenetic plants obtained from isolated microspores proved to be diploids by using a medium containing colchicine for the first 24 h of culture (Górecka et al. 2010). Andersen et al. (1990) tested the ploidy levels of 42 different clones from a carrot anther culture, and found 14 haploids, 25 diploids, and others with both haploid and diploid cells. In the study, 28 lines regenerated from the primary individual embryos or calli were established to test the ploidy level. The percentage diploidization showed huge differences among the lines and accessions (Table 4). In total, there were 68.6 % haploids, 29.9 % diploids, and 1.5 % triploids among the tested 137 plantlets. Accession 70Q78 and 80Q54 had 42.9 and 63.6 % diploids, respectively, while 70198 had only 17.5 %. Ten lines produced all haploids, 10 lines produced all diploids, and another eight lines produced different ploidy plantlets. This confirms that chromosome doubling occurs in both microspores embryogenesis and plant regeneration. An efficient chromosome doubling protocol is still required to utilize carrot haploids generated.
DH technology not only creates a true breeding line for conventional breeding programs, but also facilitates the identification of recessive and dominant mutants for genetic research. Variation and mutagenesis of plantlets were also appreciated by geneticists and breeders (Dunwell 2010; Ferrie and Möllers 2010). We obtained several plantlets that were sensitive to bolt even without any cold treatment in culture or soon after transplanting (Fig. 3a, b). Some were fertile and produced a few seeds after self-pollination (Fig. 3c), but some were sterile (Fig. 3e). In total, the leaf length of haploid plants was smaller than that of DH plants, and it was similar for DH and triploid plants (Fig. 3h, i), making visual prediction of ploidy quite efficient. Leaf color intensity and dissection of haploid plantlets varied widely even from the same accession (Fig. 3h, i).
The present study is the first report evaluating a wide range of genotypes in a carrot IMC, and a high production of calli and embryos indicates that the protocol system overcomes some shortcomings such as genotypic variation, and low frequency of microspore embryogenesis. Different ploidy plantlets regenerated from different lines demonstrated that spontaneous diploidization can occur in both carrot microspores embryogenesis and plant regeneration. The period of callus or embryo formation was much longer and rapid initiation of carrot microspore division will be one of the next steps. Given the results of this study, a carrot IMC could be effectively integrated into conventional breeding programs.
References
Adamus A, Michalik B (2003) Anther cultures of carrot (Daucus carota L.). Folia Hort 15:49–58
Ahmadi B, Alizadeh K, Teixeira da Silva JA (2012) Enhanced regeneration of haploid plantlets from microspores of Brassica napus L. using bleomycin, PCIB, and phytohormones. Plant Cell, Tissue Organ Cult 109:525–533
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Andersen SB, Christiansen I, Farestveit B (1990) Carrot (Daucus carota L.): in vitro production of haploids and field trials. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 12. Haploids in crop improvement I. Springer, Berlin, pp 393–402
Belmonte MF, Ambrose SJ, Ross ARS, Abrams SR, Stasolla C (2006) Improved development of microspore-derived embryo cultures of Brassica napus cv Topaz following changes in glutathione metabolism. Physiol Plant 127:690–700
Bhowmik P, Dirpaul J, Polowick P, Ferrie AMR (2011) A high throughput Brassica napus microspore culture system: influence of percoll gradient separation and bud selection on embryogenesis. Plant Cell, Tissue Organ Cult 106:359–362
Bradeen JM, Simon PW (2007) Carrot. In: Kole C (ed) Genome mapping and molecular breeding in plants, vol 5. Springer, Berlin, pp 161–184
Castillo AM, Cistue L, Valles MP, Soriano M (2009) Chromosome doubling in monocots. In: Touraev A, Forster B, Jain M (eds) Advances in haploid production in higher plants. Springer, Heidelberg, pp 329–338
Chang MT, Coe EH (2009) Doubled haploids. In: Kriz AL, Larkins BA (eds) Molecular genetic approaches to maize improvement. Biotechnology in agriculture and forestry, vol 63. Springer, Berlin, pp 127–142
Chen JF, Cui L, Malik AA, Mbira KG (2011) In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell, Tissue Organ Cult 104:311–319
Dohya N, Matsubara S, Murakami K (1997) Callus formation and regeneration of adventitious embryos from celery microspores by anther and isolated microspore cultures. J Jpn Soc Hortic Sci 65:747–752
Dunwell JM (2010) Haploids in flowering plants: origins and exploitation. Plant Biotechnol J 8:377–424
Fang S, Chen W, Zeng X, Zhu C, Liao X, Zheng X (2006) Isolated-microspore culture and plantlet regeneration in cabbage (Brassica oleracea L. var. capitata L.). Acta Hortic Sinica 33:158–160
Ferrie AMR, Bethune TD (2011) A microspore embryogenesis protocol for Camelina sativa, a multi-use crop. Plant Cell, Tissue Organ Cult 106:495–501
Ferrie AMR, Caswell KL (2010) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell, Tissue Organ Cult 104:301–309
Ferrie AMR, Möllers C (2010) Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell, Tissue Organ Cult 104:375–386
Ferrie AMR, Epp DJ, Keller WA (1995) Evaluation of Brassica rapa L. genotypes for microspore culture response and identification of a highly embryogenic line. Plant Cell Rep 14:580–584
Ferrie AMR, Bethune TD, Mykytyshyn M (2011) Microspore embryogenesis in Apiaceae. Plant Cell, Tissue Organ Cult 104:399–406
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirement of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Germanà MA (2006) Doubled haploid production in fruit crops. Plant Cell, Tissue Organ Cult 86:131–146
Germanà MA (2011) Anther culture for haploid and doubled haploid production. Plant Cell, Tissue Organ Cult 104:283–300
Górecka K, Krzyzanowska D, Górecki R (2005) The influence of several factors on the efficiency of androgenesis in carrot. J Appl Genet 46:265–269
Górecka K, Kowalska U, Krzyzanowska D, Kiszczak W (2010) Obtaining carrot (Daucus catota L.) plants in isolated microspore cultures. J Appl Genet 51:141–147
Grzebelus E, Szklarczyk M, Baranski R (2012) An improved protocol for plant regeneration from leaf and hypocotyl-derived protoplasts of carrot. Plant Cell, Tissue Organ Cult 109:101–109
Gu HH, Hagberg P, Zhou WJ (2004) Cold pretreatment enhances microspore embryogenesis in oilseed rape. Plant Growth Regul 42:137–143
Jauhar PP, Xu SS, Baenziger PS (2009) Haploidy in cultivated wheats: induction and utility in basic and applied research. Crop Sci 49:737–755
Kiełkowska A, Adamus A (2010) In vitro culture of unfertilized ovules in carrot (Daucus carota L.). Plant Cell, Tissue Organ Cult 102:309–319
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. E Pflanzenphysiol 105:427–434
Maluszynski M, Kasha KJ, Forster BP, Szarejko I (2003) Doubled haploid production in crop plants: a manual. Kluwer, Dordrecht
Matsubara S, Dohya N, Murakami K (1995) Callus formation and regeneration of adventitious embryos from carrot fennel and mitsuba microspores by anther and isolated microspore cultures. Acta Hortic 392:129–137
Mohammadi PP, Moieni A, Ebrahimi A, Javidfar F (2012) Doubled haploid plants following colchicine treatment of microspore-derived embryos of oilseed rape (Brassica napus L.). Plant Cell, Tissue Organ Cult 108:251–256
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Peterson CE, Simon PW (1986) Carrot breeding. In: Bassett MJ (ed) Breeding vegetable crops. AVI, Westport, pp 321–356
Seguí-Simarro J, Corral-Martínez P, Parra-Vega V, González-García B (2011) Androgenesis in recalcitrant solanaceous crops. Plant Cell Rep 30:765–778
Smith JSC, Hussain T, Jones ES, Graham G, Podlich D, Wall S, Williams M (2008) Use of doubled haploids in maize breeding: implications for intellectual property protection and genetic diversity in hybrid crops. Mol Breed 22:51–59
Smýkalová I, Šmirous P Jr, Kubošiová M, Gasmanová N, Griga M (2009) Doubled haploid production via anther culture in annual, winter type of caraway (Carum carvi L.). Acta Physiol Plant 31:21–31
Takahira J, Cousin A, Nelson MN, Cowling WA (2011) Improvement in efficiency of microspore culture to produce doubled haploid canola (Brassica napus L.) by flow cytometry. Plant Cell, Tissue Organ Cult 104:51–59
Tyukavin GB, Shmykova NA, Monahova MA (1999) Cytological study of embryogenesis in cultured carrot anthers. Russ J Plant Phys 46:876–884
Wenzel G, Bapat VA, Uhrig H (1983) New strategy to tackle breeding problems of potato. In: Sen SK, Giles KL (eds) Plant cell culture in crop improvement. Plenum Press, New York, pp 337–349
Winarto B, Teixeira da Silva JA (2011) Microspore culture protocol for Indonesian Brassica oleracea. Plant Cell, Tissue Organ Cult 107:305–315
Zhang W, Fu Q, Dai XG, Bao MZ (2008) The culture of isolated microspores of ornamental kale (Brassica oleracea var. acephala) and the importance of genotype to embryo regeneration. Sci Hortic 117:69–72
Zhuang FY, Pei HX, Ou CG, Hu H, Zhao ZW, Li JR (2010) Induction of microspores-derived embryos and calli from anther culture in carrot. Acta Hortic Sinica 37:1613–1620
Zoriniants S, Tashpulatov AS, Heberle-Bors E, Touraev A (2005) The role of stress in the induction of haploid microspore embryogenesis. In: Palmer CE, Keller WA, Kasha KJ (eds) Haploids in crop improvement. Biotechnology in Agriculture and Forestry 56, pp 35–52
Acknowledgments
This work was partially supported by the Chinese Ministry of Agriculture (948 Project) (grant number 2006-G13); the Special Fund for Agro-scientific Research in the Public Interest (grant number 200903016). Authors sincerely thank Dr. Philipp W. Simon (Wisconsin University) for kindly supplying the inbred lines of var. s. HCM A.C., BETA III, 2327, 3080B, 6366B, and 7262B, and critical review of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, JR., Zhuang, FY., Ou, CG. et al. Microspore embryogenesis and production of haploid and doubled haploid plants in carrot (Daucus carota L.). Plant Cell Tiss Organ Cult 112, 275–287 (2013). https://doi.org/10.1007/s11240-012-0235-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0235-5