Abstract
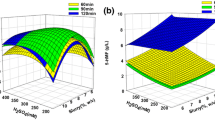
This study examined the pretreatment, enzymatic saccharification, and fermentation of the red macroalgae Gracilaria verrucosa using adapted saccharomyces cerevisiae to galactose or NaCl for the increase of bioethanol yield. Pretreatment with thermal acid hydrolysis to obtain galactose was carried out with 11.7% (w/v) seaweed slurry and 373 mM H2SO4 at 121 °C for 59 min. Glucose was obtained from enzymatic hydrolysis. Enzymatic saccharification was performed with a mixture of 16 U/mL Celluclast 1.5L and Viscozyme L at 45 °C for 48 h. Ethanol fermentation in 11.7% (w/v) seaweed hydrolysate was carried out using Saccharomyces cerevisiae KCTC 1126 adapted or non-adapted to high concentrations of galactose or NaCl. When non-adapted S. cerevisiae KCTC 1126 was used, the ethanol productivity was 0.09 g/(Lh) with an ethanol yield of 0.25. Ethanol productivity of 0.16 and 0.19 g/(Lh) with ethanol yields of 0.43 and 0.48 was obtained using S. cerevisiae KCTC 1126 adapted to high concentrations of galactose and NaCl, respectively. Adaptation of S. cerevisiae KCTC 1126 to galactose or NaCl increased the ethanol yield via adaptive evolution of the yeast.




Similar content being viewed by others
References
Bothast R, Schlicher M (2005) Biotechnological processes for conversion of corn into ethanol. Appl Microbiol Biotechnol 67(1):19–25
David C, Matteo P, Simone F, Luis O, Pieroo O, Paolo T, Francesco C (2012) Review of pretreatment processes for lignocellulosic ethanol production. Biomass Bioenergy 46:25–35
Dias MOS, Esinas AV, Nebra SA, Maciel R, Rossell CEV, Maciel MRW (2009) Production of bioethanol and other bio-based materials from sugarcane bagasse: integration to conventional bioethanol production process. Chem Eng Res Des 87:1206–1216
John RP, Anisha G, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102(1):186–193
Yanagisawa M, Kawai S, Murata K (2013) Strategies for the production of high concentrations of bioethanol from seaweeds. Bioengineered 4:224–235
Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour Technol 135:150–156
Ra CH, Jung JH, Sunwoo IY, Kang CH, Jeong GT, Kim SK (2015) Detoxification of Eucheuma spinosum hydrolysates with activated carbon for ethanol production by the salt-tolerant yeast Candia tropicalis. J Microbiol Biotechnol 25(6):856–862
Cho HY, Ra CH, Kim SK (2014) Ethanol production from the Seaweed Gelidium amansii, using specific sugar acclimated yeast. J Microbiol Biotechnol 24:264–269
Cho YK, Kim HJ, Kim SK (2013) Bioethanol production from brown seaweed, Undaria pinnatifida, using NaCl acclimated yeast. Bioprocess Biosyst Eng 36(6):713–719
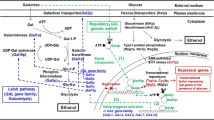
Marger WH, Siderius M (2002) Novel insights into the osmotic stress response of yeast. FEMS Yeast Res 2:251–257
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Ernandes JR, William JW, Stewart GG (1992) Simultaneous utilization of galactose and glucose by Saccharomyces spp. Biotechnol Adv 6:233–238
AOAC (Association of Official Analytical Chemists) (1995) In: Cunniff, P. (Ed.), Official methods of analysis of the association of official analytical chemists, 16th ed. Association of Official Analytical Chemists, Arlington, VA
Mendels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulose. Biotechnol Bioeng Symp 6:21–23
Kubicek CP (1982) β-glucosidase excretion by Trichoderma pseudokoningii correlation with cell wall bound β-1,3-glucanase activities. Arch Microbiol 132:349–354
Park JH, Hong JY, Jang HC, Oh SG, Kim SH, Yoon JJ, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Yoo CG, Lee CW, Kim TH (2011) Optimization of two-stage fraction process for lignocellulosic biomass using response surface methodology (RSM). Biomass Bioenergy 35:4901–4909
Kim DH, Lee SG, Jeong GT (2014) Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour Technol 161:348–353
Djioleu A, Carrier DJ (2016) Effects of dilute acid pretreatment parameters on sugar production during biochemical conversion of switchgrass using a full factorial design. ACS Sustain Chem Eng 4:4124–4130
Meinita M, Kang JY, Jeong GT, Koo H, Park S, Hong YK (2012) Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). Appl Phycol 24:857–862
Kim C, Ryu HJ, Kim SH, Yoon J-J, Kim HS, Kim YJ (2010) Acidity tunable ionic liquids as catalysts for conversion of agar into mixed sugars. Bull Korean Chem Soc 31:511–514
Wu CH, Chien WC, Chou HK, Yang J, Victor Lin HT (2014) Sulfuric acid hydrolysis and detoxification of red alga Pterocladiella capillacea for bioethanol fermentation with thermotolerant yeast Kluyveromyces marxianus. J Microbiol Biotechnol 24:1245–1253
Ra CH, Kim YJ, Lee SY, Jeong GT, Kim SK (2015) Effects of galactose adaptation in yeast for ethanol fermentation from red seaweed, Gracilaria verrucosa. Bioprocess Biosyst Eng 38:1715–1722
Wei CJ, Tanner RD, Malaney JW (1982) Effect of sodium chloride on bakers’ yeast growing in gelatin. Appl Environ Microbiol 43:757–763
Khambhaty Y, Mody K, Grandhi MR, Thampy S, Maiti P, Brambhatt H, Eswaran K, Ghosh PK (2012) Kappaphycus alvarezii as a source of bioethanol. Bioresour Technol 103:180–185
Hargreaves PI, Barcelos CA, da Costa AC, Pereira N Jr (2013) Production of ethanol 3G from Kappaphycus alvarezii: evaluation of different process strategies. Bioresour Technol 134:257–263
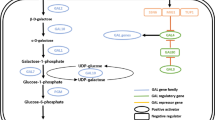
Bro C, Knudsen S, Regenberg B, Olsson L, Nielsen J (2005) Improvement of galactose uptake in Saccharomyces cerevisiae through overexpression of phosphoglucomutase: example of transcript analysis as a tool in inverse metabolic engineering. Appl Environ Microbiol 71:6465–6472
Ra CH, Choi JG, Kang CH, Sunwoo IY, Jeong GT, Kim SK (2015) Thermal acid hydrolysis pretreatment, enzymatic saccharification and ethanol fermentation from red seaweed, Gracilaria verrucosa. Microbiol Biotechnol Lett 43(1):9–15
Shuler ML, Kargi F (2002) Bioprocess engineering: basic concepts, 2nd edn. Prentice Hall, Upper Saddle River, NJ
Yan S, Wang S, Zhai Z, Chen X, Wu J (2014) Mutation breeding of salt-tolerant and ethanol-producing strain S. cerevisiae H058 by low-energy ion implantation. Adv J Food Sci Technol 6(7):941–946
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1D1A1A09918683), Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, T.H., Ra, C.H., Sunwoo, I. et al. Bioethanol production from Gracilaria verrucosa using Saccharomyces cerevisiae adapted to NaCl or galactose. Bioprocess Biosyst Eng 40, 529–536 (2017). https://doi.org/10.1007/s00449-016-1718-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1718-2