Abstract
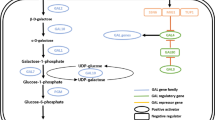
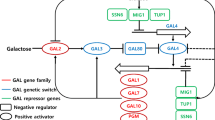
A total monosaccharide concentration of 39.6 g/L, representing 74.0 % conversion of 53.5 g/L total carbohydrate from 80 g dw/L (8 % w/v) Gracilaria verrucosa slurry, was obtained by thermal acid hydrolysis and enzymatic saccharification. G. verrucosa hydrolysate was used as a substrate for ethanol production by ‘separate hydrolysis and fermentation’ (SHF). The ethanol production and yield (Y EtOH) from Saccharomyces cerevisiae KCCM 1129 with and without adaptation to high galactose concentrations were 18.3 g/L with Y EtOH of 0.46 and 13.4 g/L with Y EtOH of 0.34, respectively. Relationship between galactose adaptation effects and mRNA transcriptional levels were evaluated with GAL gene family, regulator genes of the GAL genetic switch and repressor genes in non-adapted and adapted S. cerevisiae. The development of galactose adaptation for ethanol fermentation of G. verrucosa hydrolysates allowed us to enhance the overall ethanol yields and obtain a comprehensive understanding of the gene expression levels and metabolic pathways involved.




Similar content being viewed by others
References
Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour Technol 135:150–156
Ostergaard S, Olsson L, Johnston M, Nielsen J (2000) Increasing galactose consumption by Saccharomyces cerevisiae through metabolic engineering of the GAL gene regulatory network. Nat Biotechnol 18:1283–1286
Raamsdonk LM (2001) Co-consumption of sugars or ethanol and glucose in a Saccharomyces cerevisiae strain deleted in the HXK2 gene. Yeast 18:1023–1033
Klein CJL (1999) Investigation of the impact of MIG1 and MIG2 on the physiology of Saccharomyces cerevisiae. J Biotechnol 68:197–212
Cho HY, Ra CH, Kim SK (2014) Ethanol production from the seaweed, Gelidium amansii using specific sugar acclimated yeasts. J Microbiol Biotechnol 24:264–269
Alfani F, Gallifuoco A, Saporosi A, Spera A, Cantarella M (2000) Comparison of SHF and SSF processes for the bioconversion of steam-exploded wheat straw. J Ind Microbiol Biotechnol 25:184–192
Park JH, Hong JY, Jang HC, Oh SG, Kim SH, Yoon JJ, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Modig T, Liden G, Taherzadeh MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776
Sanchez B, Bautista J (1998) Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzyme Mircrob Technol 10:315–318
Timson DJ (2007) Galactose metabolism in Saccharomyces cerevisiae. Dyn Biochem Process Biotechnol Mol Biol 1:63–73
Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS (2012) Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol 30:274–282
Sanchez-Machado DI, Lopez-Cervantes J, Paseiro-Losada P, Lopez-Hernandez J (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85:439–444
Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotenchnol Bioeng Symp 6:21–23
Kubicek CP (1982) β-glucosidase excretion by Trichoderma pseudokoningii correlation with cell wall bound β-1,3-glucanase activities. Arch Microbiol 132:349–354
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Majumdar S, Ghatak J, Mukherji S, Bhattacharjee H, Bhaduri A (2004) UDP galactose 4-epimerase from Saccharomyces cerevisiae. A bifunctional enzyme with aldose 1-epimerase acitivity. Eur J Biochem 271:753–759
Gonçalves PM, Griffioen G, Bebelman JP, Planta RJ (1997) Signalling pathways leading to transcriptional regulation of genes involved in the activation of glycolysis in yeast. Mol Microbiol 25:483–493
Yano K, Fukasawa T (1997) Galactose-dependent reversible interaction of Gal3p with Gal80p n the induction pathway of Gal4p-activated genes of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 94:1721–1726
Verma M, Bhat PJ, Venkatesh KV (2003) Quantitative analysis of GAL genetic switch of Saccharomyces cerevisiae reveals that nucleocytoplasmic shuttling of Gal80p results in a highly sensitive response to galactose. J Biol Chem 278:48764–48769
Peng G, Hopper JE (2002) Gene activation by interaction of an inhibitor with a cytoplasmic signaling protein. Proc Natl Acad Sci USA 99:8548–8553
Wikandari R, Millati R, Syamsiyah S, Muriana R, Ayuningsih Y (2010) Effect of furfural, hydroxymethylfurfural and acetic acid on indigenous microbial isolate for bioethanol production. Agricul J 5:105–109
Palmqvist E, Hähn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2013R1A1A2059095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ra, C.H., Kim, Y.J., Lee, S.Y. et al. Effects of galactose adaptation in yeast for ethanol fermentation from red seaweed, Gracilaria verrucosa . Bioprocess Biosyst Eng 38, 1715–1722 (2015). https://doi.org/10.1007/s00449-015-1411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1411-x