Abstract
Objectives
To explore the correlation of parameters derived from intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) with Ki-67 labeling index (LI) in soft tissue sarcoma (STS).
Methods
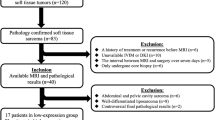
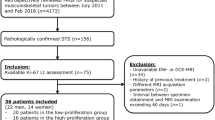
Forty-one patients with STS underwent IVIM and DKI imaging at 3.0 MRI. The standard apparent diffusion coefficient (ADC), true diffusion coefficient (D), pseudo-diffusion coefficient (D*), perfusion fraction (f), mean kurtosis (MK), and mean diffusivity (MD) were compared between Ki-67 low- and high-expression groups by two independent observers. A novel method was used to ensure the topographic correlation of histologic sections and magnetic resonance imaging slices. Receiver operating characteristic (ROC), intraclass correlation coefficient (ICC), and Spearman’s rank correlations were performed for statistical analysis.
Results
The high-expression group displayed lower standard ADC, D, and MD values and a higher MK value than the low-expression group. No significant differences were found for D∗ and f values. The areas under the curve for standard ADC, D, MD, and MK when discriminating between low- and high-expression groups were 0.736, 0.745, 0.848, and 0.894, respectively. MK was positively correlated with Ki-67 LI (r = 0.809, p < 0.001). Standard ADC, D, and MD were negatively correlated with Ki-67 LI (r = −0.541, −0.556, −0.702, respectively, p < 0.001).
Conclusions
IVIM and DKI parameters are correlated with Ki-67 LI. MK may be the optimal imaging biomarker for assessing the Ki-67 expression of STS.
Key Points
• IVIM and DKI parameters are correlated with the expression of Ki-67 in STS.
• The MRI-pathology control method ensured a strong correlation between MRI slices and histologic sections, resulting in a robust radiological-pathological correlation.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion-weighted imaging
- ICC:
-
Intraclass correlation coefficient
- IVIM:
-
Intravoxel incoherent motion
- LI:
-
Labeling index
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operating characteristic
- STS:
-
Soft tissue sarcoma
References
Gamboa A, Gronchi A, Cardona K (2020) Soft-tissue sarcoma in adults: an update on the current state of histotype-specific management in an era of personalized medicine. CA Cancer J Clin 70:200–229
Oliveira A, Nascimento AJ (2001) Grading in soft tissue tumors: principles and problems. Skeletal Radiol 30:543–559
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322
Petrelli F, Viale G, Cabiddu M, Barni S (2015) Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 153:477–491
Wen S, Zhou W, Li C et al (2015) Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: a meta-analysis of published studies involving 32 studies. BMC Cancer 15:520
Luo Y, Zhang X, Mo M et al (2016) High Ki-67 immunohistochemical reactivity correlates with poor prognosis in bladder carcinoma: a comprehensive meta-analysis with 13,053 patients involved. Medicine (Baltimore) 95(e3337):6
Zhao Y, Shen L, Huang X et al (2017) High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem Biophys Res Commun 494:390–396
Ralte A, Sharma M, Karak A, Mehta V, Sarkar C (2001) Clinicopathological features, MIB-1 labeling index and apoptotic index in recurrent astrocytic tumors. Pathol Oncol Res 7:267–278
Levine E, Holzmayer T, Bacus S et al (1997) Evaluation of newer prognostic markers for adult soft tissue sarcomas. J Clin Oncol 15:3249–3257
Rudolph P, Kellner U, Chassevent A et al (1997) Prognostic relevance of a novel proliferation marker, Ki-S11, for soft-tissue sarcoma. A multivariate study. Am J Pathol 150:1997–2007
Hasegawa T, Yokoyama R, Lee Y, Shimoda T, Beppu Y, Hirohashi S (2000) Prognostic relevance of a histological grading system using MIB-1 for adult soft-tissue sarcoma. Oncology 58:66–74
Hoos A, Stojadinovic A, Mastorides S et al (2001) High Ki-67 proliferative index predicts disease specific survival in patients with high-risk soft tissue sarcomas. Cancer 92:869–874
Lin X, Davion S, Bertsch E, Omar I, Nayar R, Laskin W (2016) Federation Nationale des Centers de Lutte Contre le Cancer grading of soft tissue sarcomas on needle core biopsies using surrogate markers. Hum Pathol 56:147–154
Yang J, Frassica F, Fayad L, Clark D, Weber K (2010) Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res 468:3103–3111
Chianca V, Albano D, Messina C et al (2021) An update in musculoskeletal tumors: from quantitative imaging to radiomics. Radiol Med 126:1095–1105
Lee J, Yoon Y, Seo S, Choi Y, Kim HS (2020) Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol 30:914–924
Le Bihan D, Breton E, Lallemand D, Aubin M, Vignaud J, Laval-Jeantet MJR (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Jensen J, Helpern J, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Wu W, Jiang G, Xu Z et al (2021) Three-dimensional pulsed continuous arterial spin labeling and intravoxel incoherent motion imaging of nasopharyngeal carcinoma: correlations with Ki-67 proliferation status. Quant Imaging Med Surg 11:1394–1405
Wang F, Wu L, Hua X, Zhao Z, Chen X, Xu JR (2018) Intravoxel incoherent motion diffusion-weighted imaging in assessing bladder cancer invasiveness and cell proliferation. J Magn Reson Imaging 47:1054–1060
Zheng Y, Huang W, Zhang X, et a (2021) A noninvasive assessment of tumor proliferation in lung cancer patients using intravoxel incoherent motion magnetic resonance imaging. J Cancer 12:190-197
Huang Y, Lin Y, Hu W et al (2019) Diffusion kurtosis at 3.0T as an in vivo imaging marker for breast cancer characterization: correlation with prognostic factors. J Magn Reson Imaging 49:845–856
Xiao Z, Zhong Y, Tang Z et al (2018) Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 28:2923–2933
Yuan Y, Zeng D, Liu Y et al (2020) DWI and IVIM are predictors of Ki67 proliferation index: direct comparison of MRI images and pathological slices in a murine model of rhabdomyosarcoma. Eur Radiol 30:1334–1341
Choi J, Ro J (2021) The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol 28:44–58
Li X, Liu Y, Tao J et al (2021) Value of intravoxel incoherent motion and diffusion kurtosis imaging in predicting peritumoural infiltration of soft-tissue sarcoma: a prospective study based on MRI-histopathology comparisons. Clin Radiol 76:532–539
Li X, Yang L, Wang Q, Tao J, Pan Z, Wang S (2021) Soft tissue sarcomas: IVIM and DKI correlate with the expression of HIF-1α on direct comparison of MRI and pathological slices. Eur Radiol 31:4669–4679
Chhabra A, Ashikyan O, Slepicka C et al (2019) Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 29:4485–4494
Arslan S, Ergen FB, Aydın GB et al (2021) Different attenuation models of diffusion-weighted MR imaging for the differentiation of benign and malignant musculoskeletal tumors. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27887
Kim S, Cha E, Kim H et al (2009) Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 30:615–620
Song X, Wang L, Ren H, Wei R, Ren J, Niu J (2020) Intravoxel incoherent motion imaging in differentiation borderline from malignant ovarian epithelial tumors: correlation with histological cell proliferation and vessel characteristics. J Magn Reson Imaging 51:928–935
Pang Y, Turkbey B, Bernardo M et al (2013) Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med 69:553–562
Koh D, Collins D, Orton M (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196:1351–1361
Dyvorne H, Galea N, Nevers T et al (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology 266:920–929
Liu C, Wang K, Chan Q et al (2016) Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol. 26:3888–3898
Liu Y, Yin Z, Li X et al (2021) The diagnostic accuracy of intravoxel incoherent motion and diffusion kurtosis imaging in the differentiation of malignant and benign soft-tissue masses: which is better? Acta Radiol:2841851211017511
Ogawa M, Kan H, Arai N et al (2019) Differentiation between malignant and benign musculoskeletal tumors using diffusion kurtosis imaging. Skeletal Radiol 48:285–292
Zhang J, Chen X, Chen D, Wang Z, Li S, Zhu W (2018) Grading and proliferation assessment of diffuse astrocytic tumors with monoexponential, biexponential, and stretched-exponential diffusion-weighted imaging and diffusion kurtosis imaging. Eur J Radiol 109:188–195
Zhao F, Ahlawat S, Farahani S et al (2014) Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 272:192–201
Crombé A, Marcellin P, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology 291:710–721
Funding
This study has received funding by National Natural Science Foundation of China (81771804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Institutional Ethics Committee of the second Hospital of Dalian Medical University.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Conflict of interest
One of the authors (Lizhi Xie) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Guarantor
The scientific guarantor of this publication is Shaowu Wang, MD, PHD.
Statistics and biometry
One of the authors has significant statistical expertise.
Methodology
• prospective
• case-control study/diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Dai, Y., Liu, Y. et al. Soft tissue sarcoma: IVIM and DKI parameters correlate with Ki-67 labeling index on direct comparison of MRI and histopathological slices. Eur Radiol 32, 5659–5668 (2022). https://doi.org/10.1007/s00330-022-08646-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08646-1