Abstract
Objectives
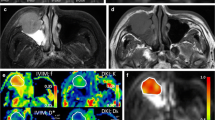
To explore the correlations of parameters derived from standard diffusion-weighted imaging (DWI), diffusion kurtosis imaging (DKI) and intravoxel incoherent motion (IVIM) with the Ki-67 proliferation status.
Methods
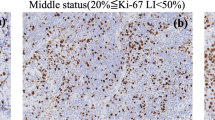
Seventy-five patients with histologically proven sinonasal malignancies who underwent standard DWI, DKI and IVIM were retrospectively reviewed. The mean, minimum, maximum and whole standard DWI [apparent diffusion coefficient (ADC)], DKI [diffusion kurtosis (K) and diffusion coefficient (Dk)] and IVIM [pure diffusion coefficient (D), pseudo-diffusion coefficient (D*) and perfusion fraction (f)] parameters were measured and correlated with the Ki-67 labelling index (LI). The Ki-67 LI was categorised as high (> 50%) or low (≤ 50%).
Results
The K and f values were positively correlated with the Ki-67 LI (rho = 0.295~0.532), whereas the ADC, Dk and D values were negatively correlated with the Ki-67 LI (rho = -0.443~-0.277). The ADC, Dk and D values were lower, whereas the K value was higher in sinonasal malignancies with a high Ki-67 LI than in those in a low Ki-67 LI (all p < 0.05). A higher maximum K value (Kmax > 0.977) independently predicted a high Ki-67 status [odds ratio (OR) = 7.614; 95% confidence interval (CI) = 2.197-38.674; p = 0.017].
Conclusion
ADC, Dk, K, D and f are correlated with Ki-67 LI. Kmax is the strongest independent factor for predicting Ki-67 status.
Key Points
• DWI-derived parameters from different models are capable of providing different pathophysiological information.
• DWI, DKI and IVIM parameters are associated with Ki-67 proliferation status.
• K max derived from DKI is the strongest independent factor for the prediction of Ki-67 proliferation status.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion-weighted imaging
- EPI:
-
Echo planar imaging
- FOV:
-
Field of view
- ICC:
-
Intraclass correlation coefficient
- IVIM:
-
Intravoxel incoherent motion
- LI:
-
Labelling index
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PPV:
-
Positive predictive value
- ROC:
-
Receiver-operating characteristic
- ROIs:
-
Regions of interest
References
Slootweg PJ, Ferlito A, Cardesa A et al (2013) Sinonasal tumors: a clinicopathologic update of selected tumors. Eur Arch Otorhinolaryngol 270:5–20
Su SY, Kupferman ME, DeMonte F et al (2014) Endoscopic resection of sinonasal cancers. Curr Oncol Rep 16:369
Eggesbo HB (2012) Imaging of sinonasal tumours. Cancer Imaging 12:136–152
Koeller KK (2016) Radiologic features of sinonasal tumors. Head Neck Pathol 10:1–12
Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T (2001) Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer 92:3012–3029
Bhattacharyya N (2002) Cancer of the nasal cavity: survival and factors influencing prognosis. Arch Otolaryngol Head Neck Surg 128:1079–1083
Valente G, Mamo C, Bena A et al (2006) Prognostic significance of microvessel density and vascular endothelial growth factor expression in sinonasal carcinomas. Hum Pathol 37:391–400
Airoldi M, Garzaro M, Valente G et al (2009) Clinical and biological prognostic factors in 179 cases with sinonasal carcinoma treated in the Italian Piedmont region. Oncology 76:262–269
Chen WJ, He DS, Tang RX, Ren FH, Chen G (2015) Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev 16:411–420
Stathopoulos GP, Malamos NA, Markopoulos C et al (2014) The role of Ki-67 in the proliferation and prognosis of breast cancer molecular classification subtypes. Anticancer Drugs 25:950–957
Fukushima S, Sugita Y, Niino D, Mihashi H, Ohshima K (2012) Clincopathological analysis of olfactory neuroblastoma. Brain Tumor Pathol 29:207–215
Le Bihan D (1995) Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed 8:375–386
Yan R, Haopeng P, Xiaoyuan F et al (2016) Non-Gaussian diffusion MR imaging of glioma: comparisons of multiple diffusion parameters and correlation with histologic grade and MIB-1 (Ki-67 labeling) index. Neuroradiology 58:121–132
Shin JK, Kim JY (2017) Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 45:94–102
Li HM, Zhao SH, Qiang JW et al (2017) Diffusion kurtosis imaging for differentiating borderline from malignant epithelial ovarian tumors: a correlation with Ki-67 expression. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25696
Driessen JP, Caldas-Magalhaes J, Janssen LM et al (2014) Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: association between apparent diffusion coefficient and histologic findings. Radiology 272:456–463
Yuan J, Yeung DK, Mok GS et al (2014) Non-Gaussian analysis of diffusion weighted imaging in head and neck at 3T: a pilot study in patients with nasopharyngeal carcinoma. PLoS One 9:e87024
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Le Bihan D (1988) Intravoxel incoherent motion imaging using steady-state free precession. Magn Reson Med 7:346–351
Le Bihan D, Breton E, Lallemand D et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Jiang JX, Tang ZH, Zhong YF, Qiang JW (2016) Diffusion kurtosis imaging for differentiating between the benign and malignant sinonasal lesions. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25500
Sumi M, Nakamura T (2013) Head and neck tumors: assessment of perfusion-related parameters and diffusion coefficients based on the intravoxel incoherent motion model. AJNR Am J Neuroradiol 34:410–416
Sumi M, Nakamura T (2014) Head and neck tumours: combined MRI assessment based on IVIM and TIC analyses for the differentiation of tumors of different histological types. Eur Radiol 24:223–231
Sumi M, Van Cauteren M, Sumi T et al (2012) Salivary gland tumors: use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 263:770–777
Lu Y, Jansen JF, Mazaheri Y et al (2012) Extension of the intravoxel incoherent motion model to non-Gaussian diffusion in head and neck cancer. J Magn Reson Imaging 36:1088–1096
Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A (2010) Non-Gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: a feasibility study. AJNR Am J Neuroradiol 31:741–748
Sun K, Chen X, Chai W et al (2015) Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277:46–55
Le Bihan D, Turner R, MacFall JR (1989) Effects of intravoxel incoherent motions (IVIM) in steady-state free precession (SSFP) imaging: application to molecular diffusion imaging. Magn Reson Med 10:324–337
Marzi S, Piludu F, Vidiri A (2013) Assessment of diffusion parameters by intravoxel incoherent motion MRI in head and neck squamous cell carcinoma. NMR Biomed 26:1806–1814
Fujima N, Yoshida D, Sakashita T et al (2017) Prediction of the treatment outcome using intravoxel incoherent motion and diffusional kurtosis imaging in nasal or sinonasal squamous cell carcinoma patients. Eur Radiol 27:956–965
Fudaba H, Shimomura T, Abe T et al (2014) Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 35:2091–2098
Iima M, Le Bihan D (2016) Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278:13–32
Le Bihan D, Turner R (1992) The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 27:171–178
Lewin M, Fartoux L, Vignaud A et al (2011) The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol 21:281–290
Liu C, Wang K, Chan Q et al (2016) Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 26:3888–3898
Lai V, Lee VH, Lam KO et al (2015) Intravoxel water diffusion heterogeneity MR imaging of nasopharyngeal carcinoma using stretched exponential diffusion model. Eur Radiol 25:1708–1713
Parikh J, Selmi M, Charles-Edwards G et al (2014) Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology 272:100–112
Yoon SH, Park CM, Park SJ et al (2016) Tumor heterogeneity in lung cancer: assessment with dynamic contrast-enhanced MR imaging. Radiology 280:940–948
Funding
This study has received funding from the Grant of Science and Technology Commission of Shanghai Municipality (no. 17411962100; 14411962000) and Shanghai Municipal Commission of Health and Family Planning (grant no. ZK2015A05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Zuohua Tang, MD, PhD, Eye and ENT Hospital of Shanghai Medical School, Fudan University, and Prof. Jinwei Qiang, MD, PhD, Jinshan Hospital of Shanghai Medical School, Fudan University.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Xiao, Z., Zhong, Y., Tang, Z. et al. Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 28, 2923–2933 (2018). https://doi.org/10.1007/s00330-017-5286-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5286-x