Abstract
Background
Chronic hepatitis B (CHB) virus infection is a major global healthcare problem. The recent introduction of entecavir in Australia for the treatment of CHB patients in the naive treatment setting has triggered significant optimism with regards to improved clinical outcomes for CHB patients.
Objective
To estimate, from an Australian healthcare perspective, the cost effectiveness of entecavir 0.5mg/day versus lamivudine 100mg/day in the treatment of CHB patients naive to nucleos(t)ide therapy.
Methods
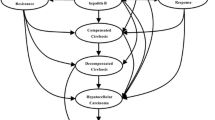
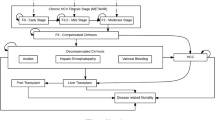
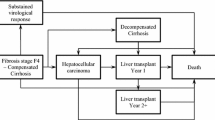
A cost-utility analysis to project the clinical and economic outcomes associated with CHB disease and treatment was conducted by developing two decision-tree models specific to hepatitis B e antigen-positive (HBeAg+ve) and HBeAg−ve CHB patient subsets. This analysis was constructed using the Australian payer perspective of direct costs and outcomes, with indirect medical costs and lost productivity not being included. The study population comprised a hypothetical cohort of 1000 antiviral treatment-naive CHB patients who received either entecavir 0.5mg/day or lamivudine 100 mg/day at model entry. The population of patients used in this analysis was representative of those patients likely to receive initial antiviral therapy in clinical practice in Australia. The long-term cost effectiveness of entecavir compared with lamivudine in the first-line treatment of CHB patients was expressed as an incremental cost per life-year gained (LYG) or QALY gained.
Results
Results revealed that the availability of entecavir 0.5mg/day as part of the Australian hepatologist’s treatment armamentarium should result in significantly lower future rates of compensated cirrhosis (CC), decompensated cirrhosis (DC), and hepatocellular carcinoma (HCC) events (i.e. 54 fewer cases of CC, seven fewer cases of DC, and 20 fewer cases of HCC over the model’s timeframe for HBeAg+ve CHB patients, and 69 fewer cases of CC, eight fewer cases of DC and 25 fewer cases of HCC over the model’s timeframe for HBeAg−ve CHB patients). Compared with lamivudine 100 mg/day, entecavir 0.5 mg/day generated an estimated incremental cost per LYG of Australian dollars ($A, year 2006 values) 5046 and an estimated incremental cost per QALY of $A5952 in the HBeAg+ve CHB patient population, an estimated incremental cost per LYG of $A7063 and an estimated incremental cost per QALY of $A8003 in the HBeAg−ve CHB patient population, and an overall estimated incremental cost per LYG of $A5853 and an estimated incremental cost per QALY of $A6772 in the general CHB population.
Conclusion
The availability of entecavir in Australian clinical practice should make long-term suppression of hepatitis B virus replication increasingly attainable, resulting in fewer CHB sequelae, at an acceptable financial cost.










Similar content being viewed by others
Notes
1Telbivudine is the most recent antiviral drug approved by the Pharmaceutical Benefits Advisory Committee (PBAC) for the treatment of CHB (PBS listed December 2008). However, telbivudine is not included in this analysis as the PBAC recommendation for telbivudine (HBeAg+ve CHB patients only) is narrower than that of entecavir and lamivudine (HBeAg+ve and HBeAg−ve CHB patients).
References
Andre F. Hepatitis B: a comprehensive prevention, diagnosis and treatment program. Past, present and future. J Gastroenterol Hepatol 2004; 19: S1–4
Alter MJ. Epidemiology and prevention of hepatitis B. Sem Liver Dis 2003; 23: 39–46
Williams A. Reduction in the hepatitis B related burden of disease: measuring the success of universal immunisation programs. Commun Dis Intell 2002; 26: 458–60
Dore G, Wallace J, Locarnini S, et al. Hepatitis B in Australia: responding to a diverse epidemic. ACT-HBV (Advancing the Clinical Treatment of Hepatitis B Virus) [online]. Available from URL: http://alliance.hepatitis.org.au/uploads/ACT_HBV.pdf [Accessed 2006 Jan 23]
Papatheodoridis GV, Dimou E, Papadimitropoulos V. Nucleos(t)ide analogues for CHB: antiviral efficacy and viral resistance. Am J Gastroenterol 2002; 97: 1618–28
Conjeevaram HS, Lok AS. Management of CHB. J Hepatol 2003; Suppl. 31: S90–103
Papatheodoridis GV, Hadziyannis SJ. Current management of CHB. Aliment Pharmacol Ther 2004; 19: 25–37
Leung NW, Lai CL, Chang TT, et al. Extended lamivudine treatment in patients with CHB enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33: 1527–32
Andreone P, Gramenzi A, Cursaro C, et al. High risk of hepatocellular carcinoma in anti-HBe positive liver cirrhosis patients developing lamivudine resistance. J Viral Hepat 2004; 11: 439–42
Lavanchy D. Hepatitis B: viral epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11: 97–107
Gish RG. Clinical trial results of new therapies for HBV: implications for treatment guidelines. Sem Liver Dis 2005; 25: 29–39
Perrillo RP. Therapy of hepatitis B: viral suppression or eradication. Hepatology 2006; 43(2 Suppl. 1): S182–S193
Jacobson I. Hepatitis B: science and treatment. DDW 2005 Conference Report [online]. Available from URL: http://www.natap.org/2005/ddw/ddw.htm [Accessed 2006 Jan 23]
Yim HJ, Lok AS. Natural history of CHB virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006; 43: S173–81
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. NEJM 2006; 354: 1001–10
Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. NEJM 2006; 354: 1011–20
Yuan Y, Iloeje U, Hau J, et al. Evaluation of the cost-effectiveness of entecavir versus lamivudine in hepatitis BeAg-positive chronic hepatitis B patients. J Manag Care Pharm 2008; 14: 21–33
Yuan Y, Iloeje U, Li H, et al. Economic implications of entecavir treatment in suppressing viral replication in chronic hepatitis B (CHB) patients in China from a perspective of the Chinese Social Security Program. Value Health 2008; 11(Suppl. 1): S11–22
Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295: 65–73
Iloeje UH, Yang SI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130: 678–86
EASL Jury. EASL International Consensus Conference on Hepatitis B: consensus Statement. J Hepatol 2003; 39: S3–25
Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int 2005; 25: 472–89
Lok SF, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–39
Keeffe EB, Dieterich DT, Han SB, et al. A treatment algorithm for the management of CHB virus infection in the United States. Clin Gastroenterol Hepatol 2004; 2: 87–106
Sherman M, Yurdaydin C, Sollano J, et al. Entecavir for treatment of lamivudine-refractory, HBEAg-positive chronic hepatitis B. Gastroenterology 2006; 130: 2039–49
Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. J Hepatol 2006; 44: 1656–65
Colonno RJ, Rose RE, Pokornowski K, et al. Four year assessment of entecavir resistance in nucleoside-naive and lamivudine refractory patients. J Hepatol 2007; 46(Suppl. 1): S294
Tenney DJ, Levine SM, Rose RE, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrobial Agents Chemother 2004; 48: 3498–507
Osiowy C, Heathcote J, Zoulim F, et al. Resistance to adefovir dipivoxil in patients with lamivudine-resistant chronic hepatitis B [abstract]. Hepatology 2005; 42, S1 (p586A): 993
Lee Y, Suh D, Lim Y, et al. Emergence of rtA181V/Tand rtN236T mutations after 48 weeks of adefovir dipivoxil therapy in patients with lamivudine-resistant chronic hepatitis B [abstract]. Hepatology 2005; 42, S1 (p578A): 972
Lee C, Yeon J, Hong S, et al. More frequent and earlier emergence of adefovir resistance mutations in lamivudine resistant patients treated with ADV compared to previously reported nucleos(t)ide-treatment naive patients [abstract]. Hepatology 2005; 42, S1 (p593A): 1009
Tenney DJ, Pokorowski KA, Rose RE, et al. Entecavir at 5 years shows long term maintenance of high genetic barrier to hepatitis B virus resistance. APASL Oral Presentation 2008 [online]. Available from URL: http://natap.org/2008/HBV/032508_04.htm [Accessed 2006 Jan 23]
Gish RG, Lok SS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg positive chronic hepatitis B. Gastroenterology 2007; 133: 1347–54
Peters MG, Hann HW, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant CHB. Gastroenterology 2004; 126: 91–101
Angus P, Locarnini S. Lamivudine-resistant hepatitis B virus and ongoing lamivudine therapy: stop the merry-go-round, it’s time to get off! Antivir Ther 2004; 9: 145–8
Bell SJ, Lau A, Thompson A, et al. Chronic hepatitis B: recommendations for therapy based on the natural history of disease in Australian patients. J Clin Virol 2005; 32: 122–7
Yang HI, Iloeje UH, SU J, et al. Progression to decompensated cirrhosis in chronic hepatitis B virus infected persons is strongly associated with baseline viral load [poster]. 40th European Society for the Study of Liver Meeting; 2005 Apr 13-17; Paris. J Hepatol 2005; 42: 195
Australian Bureau of Statistics (ABS). Life Tables, Australia, 2004-2006. ABS, cat. no. 3302.0.55.001[online]. Available from URL: http://www.ausstats.abs.gov.au/ausstats/abs@archive.nsf/0/A272A6537B9EFD9DCA25738D000F2323/$File/3302_0_55_001_2006.xls [Accessed 2008 Jun 18]
Durand-Zaleski I, Zaleski S. DEALE-ing and discounting: a simple way to compute the accrued costs of preventative strategies. Med Decis Making 1994; 14: 98–103
Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost-effectiveness analysis. Gut 2001; 48: 251–9
Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002; 97: 2886–95
Levy AR, Kowdley KV, Iloeje U, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health 2008; 11(3): 527–38
Australian Government Schedule of Pharmaceutical Benefits [online]. Available from URL: http://www.pbs.gov.au/html/industry/home [Accessed 2008 Jun 16]
Butler JRG, Piank S, Korda RJ, et al. The direct cost of managing patients with CHB infection in Australia. J Clin Gastroenterol 2004; 30: 187–92
Mohamed R, Desmond P, Dong-Jin S, et al. Practical difficulties in the management of hepatitis B in the Asia-Pacific region. J Gastroenterol Hepatol 2004; 19: 958–69
O’sullivan BG, Gidding HF, Law M, et al. Estimates of CHB virus infection in Australia, 2000. Aust N Z J Pub Health 2004; 28: 212–6
Amin J, Law MG, Bartlett M, et al. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet 2006; 368: 938–45
The Public Health Association of Australia. Hepatitis B vaccination [online]. Available from URL: http://www.phaa.net.au/policy/HepBF.htm [Accessed 2005 Oct]
Department of Health and Aging. The Australian immunisation handbook. 8th ed., p. 130 [online]. Available from URL: http://www.immunise.health.gov.au/handbook.htm [Accessed 2005 Oct 19]
Ohkubo K, Kato Y, Ichikawa T, et al. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer 2002; 94: 2663–8
Mommeja-Manin H, Mondou E, Blum MR, et al. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology 2003; 37: 1309–19
Ohata K, Hamasaki K, Toriyama K, et al. High viral load is a risk factor for hepatocellular carcinoma in patients with CHB virus infection. J Gastroenterol Hepatol 2004; 19: 670–5
Yu MW, Yeh SH, Chen PJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97: 265–72
Krajden M, McNabb G, Petric M. The laboratory diagnosis of hepatitis B virus. Can J Infect Dis Med Microbiol 2005; 16: 65–72
Marco VD, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40: 883–91
Acknowledgements
The authors are grateful to Associate Professor Greg Dore (Head, Viral Hepatitis Clinical Research Program, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, NSW, Australia) for his insightful comments regarding this manuscript.
This work was funded by Bristol-Myers Squibb Australia Pty Ltd. All authors are employees of Bristol-Myers Squibb. Ms Arnold, Dr Iloeje and Dr Cook own stock in Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arnold, E., Yuan, Y., Iloeje, U. et al. Cost-effectiveness analysis of entecavir versus lamivudine in the first-line treatment of Australian patients with chronic hepatitis B. Appl Health Econ Health Policy 6, 231–246 (2008). https://doi.org/10.1007/BF03256136
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256136