Abstract
Background
Chronic Hepatitis C virus (cHCV) is a major health issue worldwide. New effective direct-acting anti-viral (DAA) drugs such as the combination sofosbuvir/velpatasvir, represent an important turning point, given the high sustained virologic response (SVR) rates associated with their use.
Objectives
To estimate the cost and effects of two different treatment strategies based on sofosbuvir/velpatasvir. Strategy 1: treating all patients, including those in the early stages of fibrosis; Strategy 2: reserving treatments for patients at more advanced stages of disease (≥ F3). The analysis compares the incremental cost-effectiveness ratio (ICER) of Strategy 1 versus Strategy 2 in a cohort of HCV-infected patients and a cohort of hepatitis C virus (HCV)-human immunodeficiency virus (HIV) patients.
Methods
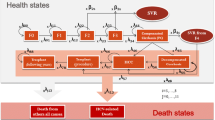
A Markov model simulating the natural history of the disease was built considering a 60-year time horizon and two cohorts of 1000 patients aged ≥ 35 years. Disease morbidity was classified according to the METAVIR classification. The robustness of the model was tested using deterministic and probabilistic sensitivity analyses (PSA).
Results
In both cohorts, Strategy 1 results in higher resource consumption and a greater number of quality-adjusted life-years (QALYs) compared with Strategy 2. The ICERs for the cohort of HCV patients and the cohort of co-infected HCV/HIV patients ranged between €15,555–74,804/QALY and €10,708–55,138/QALY, respectively, depending on the assumed cost of the treatment. In the PSA, the ICER distribution remained below the threshold of €30,000/QALY in 96 and 97% of the scenarios in the cohorts of HCV and HCV/HIV patients, respectively.
Conclusions
Extending the treatment of HCV to patients at an early stage of HCV infection is estimated to be cost effective from the perspective of the Italian Healthcare System.







Similar content being viewed by others
Notes
Concerning the patients with genotype 3 affected by compensated cirrhosis, the addition of RBV might be considered [Source: Epclusa Product Characteristics Summary].
References
World Health Organization’s Website. Available at: http://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed Mar 2016.
MSD Italia. Available at: http://www.msd-italia.it/sala_stampa/cartelle/CSHIV&HCV.pdf. Accessed Mar 2016.
Poynard T, Ratziu V, Benmanov Y, Di Martino V, Bedossa P, Opolon P. Fibrosis in patients with chronic hepatitis C: detection and significance. In: Seminars in liver disease, vol 20, no. 01. New York: Copyright© 2000 by Thieme Medical Publishers, Inc.; 2000. p. 0047–0056.
Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in HIV and HCV coinfected patients. The MULTIVIRC Group. Hepatology. 1999;30:1054–8.
Peter L, et al. AIDS. 2012;26(15):1917–26.
Freiberg MS, et al. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32.
Younossi ZM, Stepanova M, Afdhal N, Kowdley KV, Zeuzem S, Henry L, Marcellin P, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol. 2015;63(2):337–45.
Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S, ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–607.
Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M, ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–17.
Cenderello G, Artioli S, Viscoli C, Pasa A, Giacomini M, Giannini B, Dentone C, Nicolini LA, Cassola G, Di Biagio A. Budget impact analysis of sofosbuvir-based regimens for the treatment of HIV/HCV-coinfected patients in northern Italy: a multicenter regional simulation. Clinicoecon Outcomes Res. 2015;31(8):15–21.
Ruggeri M, Coretti S, Gasbarrini A, Cicchetti A. Economic assessment of an anti-HCV screening program in Italy. Value Health. 2013;16(6):965–72.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Mak. 1993;13:322–38.
Alagoz O, Hsu H, Schaefer AJ, Roberts MS. Markov decision processes: a tool for sequential decision making under uncertainty. Med Decis Mak. 2010;30(4):474–83.
Dienstag JL, Ghany MG, Morgan TR, HALT-C Trial Group, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54(2):396–405.
Kondili LA, Romano F, Rolli FR, Ruggeri M, Rosato S, Brunetto MR, Zignego AL, Ciancio A, Di Leo A, Raimondo G, Ferrari C, Taliani G, Borgia G, Santantonio TA, Blanc P, Gaeta GB, Gasbarrini A, Chessa L, Erne EM, Villa E, Ieluzzi D, Russo FP, Andreone P, Vinci M, Coppola C, Chemello L, Madonia S, Verucchi G, Persico M, Zuin M, Puoti M, Alberti A, Nardone G, Massari M, Montalto G, Foti G, Rumi MG, Quaranta MG, Cicchetti A, Craxì A, Vella S, PITER Collaborating Group. Modeling cost-effectiveness and health gains of a “universal” versus “prioritized” hepatitis C virus treatment policy in a real-life cohort. Hepatology. 2017;66(6):1814–25.
Sáez-Royuela F, Badia E. Hepatitis c: is regression of advanced fibrosis possible after treatment? EMJ. 2016;1(3):126–32.
Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56(5):1171–80.
Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomized controlled trial and economic evaluation. Health Technol Assess (Winchester, England). 2006;10(4):1–250.
Campos NG, Salomon JA, Servoss JC, Nunes DP, Samet JH, Freedberg KA, Goldie SJ. Cost-effectiveness of treatment for hepatitis C in an urban cohort co-infected with HIV. Am J Med. 2007;120(3):272–9.
Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–8.
Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–40.
Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14(8):1068–77.
Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, Wong JB, et al. Cost effectiveness of peginterferon α-2b plus ribavirin versus interferon α-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425–32.
Schwarzinger M, Dewedar S, Rekacewicz C, Elaziz KMA, Fontanet A, Carrat F, Mohamed MK. Chronic hepatitis C virus infection: does it really impact health-related quality of life? A study in rural Egypt. Hepatology. 2004;40(6):1434–41.
Ruggeri M, Coretti S, Romano F, Kondili L, Vella S, Cicchetti A. Economic evaluation of the HCV treatment extension to early-stage fibrosis patients: evidence from the PITER real-world cohort. Value Health. 2017.
Ministero della Salute Decreto 18 ottobre 2012. Allegato 1. Remunerazione delle prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale.
Sullivan SD, Craxi A, Alberti A, Giuliani G, De Carli C, Wintfeld N, Green J. Rapporto costo-efficacia della terapia peginterferone α-2a+ ribavirina in confronto a interferone α-2b+ ribavirina in pazienti affetti da epatite cronica di tipo C precedentemente non trattati. PharmacoEcon Italian Res Articles. 2004;6(2):105–14.
Ruggeri M, Basile M, Coretti S, Drago C, Cicchetti A. Economic analysis and budget impact of tenofovir and entecavir in the first-line treatment of hepatitis B virus in Italy. Appl Health Econ Health Policy. 2017;15(4):479–90.
Liberato N, et al. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2007;25(6):625–33.
Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton M, ASTRAL-4 Investigators. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–28.
Tamori A, Enomoto M, Kawada N. Recent advances in antiviral therapy for chronic hepatitis C. Mediat Inflamm. 2016;2016:6841628.
Kondili LA, Gaeta GB, Ieluzzi D, Zignego AL, Monti M, Gori A, et al. Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS One. 2017;12(2):e0172159.
Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61(6):1860–9.
Deterding K, Grüner N, Buggisch P, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013;13(6):497–506.
Obach D, Yazdanpanah Y, Esmat G, Avihingsanon A, Dewedar S, Durier N, Eholié SP. How to optimize hepatitis C virus treatment impact on life years saved in resource-constrained countries. Hepatology. 2015;62(1):31–9.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to the conception, design, acquisition of data and related analysis and interpretation and participated in drafting the article and revising it critically and according to its important intellectual content.
Corresponding author
Ethics declarations
Data availability statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Funding
This study was funded by Gilead Sciences Srl. The views expressed here are those of the authors and not necessarily those of the funders.
Conflict of interest
None of the authors (Dr. Ruggeri, Drs. Romano F, Dr. Basile, Drs. Coretti, Drs. Rolli, Dr. Drago and Dr. Cicchetti) has any potential conflict of interest related to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruggeri, M., Romano, F., Basile, M. et al. Cost-Effectiveness Analysis of Early Treatment of Chronic HCV with Sofosbuvir/Velpatasvir in Italy. Appl Health Econ Health Policy 16, 711–722 (2018). https://doi.org/10.1007/s40258-018-0410-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0410-x