Abstract
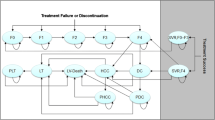
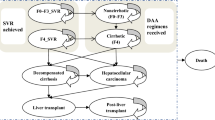
The WHO estimates that more than 185 million people are infected with hepatitis C virus (HCV) worldwide. The aim of the study is to assess the incremental cost-effectiveness ratio (ICER) of the use of daclatasvir (DCV) + sofosbuvir (SOF) + ribavirin (RBV) for 12 and 16 weeks vs SOF + RBV for 16 and 24 weeks for the treatment of genotype 3 HCV infected cirrhotic patients from the Italian National Health Service (NHS) perspective. A published cohort-based Markov model was used to perform the analysis estimating the lifetime direct medical costs associated with the management of the pathology and the quality adjusted life years gained by patients. Deterministic and probabilistic sensitivity analyses were performed to test the robustness of the results. SOF + RBV for 16 weeks was excluded from the analysis due to the significant lower effectiveness, compared with SOF + RBV for 24 weeks (51% vs 79%). DCV + SOF + RBV would increase QALYs and costs in all the comparisons: the ICERs obtained comparing DCV + SOF + RBV for 12 and 16 weeks with SOF + RBV for 24 weeks (reference scenario) are 38,572 €/QALY and 16,436 €/QALY, respectively, both below the 40,000 €/QALY threshold identified by the Italian Health Economics Association. Sensitivity analyses confirmed the robustness of the results. The use of DCV + SOF + RBV is likely to be cost-effective compared with SOF + RBV (for 24 weeks) for the treatment of cirrhotic patients infected with genotype 3 HCV considering a threshold value of 40,000 €/QALY.


Similar content being viewed by others
References
World Health Organization.: Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: World Health Organization; 2014 Apr. Available at: http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1&ua=1
Lavanchy, D.: Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17(2), 107–115 (2011)
Westbrook, R.H., Dusheiko, G.: Natural history of hepatitis C. J. Hepatol. 61(1 Suppl), S58–S68 (2014)
Cenci, M., Massi, M., Alderisio, M., De Soccio, G., Recchia, O.: Prevalence of hepatitis C virus (HCV) genotypes and increase of type 4 in central Italy: an update and report of a new method of HCV genotyping. Anticancer Res. 27(2), 1219–1222 (2007)
Pizzillo, P., Almasio, P.L., Ferraro, D., Craxì, A., Di Stefano, R.: HCV genotypes in Sicily: is there any evidence of a shift? J. Med. Virol. 81(6), 1040–1046 (2009)
Liberto, M.C., Marascio, N., Zicca, E., Matera, G.: Epidemiological features and specificities of HCV infection: a hospital-based cohort study in a university medical center of Calabria region. BMC Infect. Dis. 12(Suppl 2), S4 (2012)
Ansaldi, F., Bruzzone, B., Salmaso, S., Rota, M.C., Durando, P., Gasparini, R., Icardi, G.: Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J. Med. Virol. 76(3), 327–332 (2005)
Nkontchou, G., Ziol, M., Aout, M., Lhabadie, M., Baazia, Y., Mahmoudi, A., Roulot, D., Ganne-Carrie, N., Grando-Lemaire, V., Trinchet, J.C., Gordien, E., Vicaut, E., Baghad, I., Beaugrand, M.: HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 18(10), e516–e522 (2011)
Bochud, P.Y., Cai, T., Overbeck, K., Bochud, M., Dufour, J.F., Müllhaupt, B., Borovicka, J., Heim, M., Moradpour, D., Cerny, A., Malinverni, R., Francioli, P., Negro, F., Swiss Hepatitis C Cohort Study Group: Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J. Hepatol. 51(4), 655–666 (2009)
Majumdar, A., Kitson, M.T., Roberts, S.K.: Treatment of hepatitis C in patients with cirrhosis: remaining challenges for direct-acting antiviral therapy. Drugs 75(8), 823–834 (2015)
Kattakuzhy, S., Levy, R., Rosenthal, E., Tang, L., Wilson, E., Kottilil, S.: Hepatitis C genotype 3 disease. Hepatol. Int. (2016) (Epub ahead of print)
Pol, S., Vallet-Pichard, A., Corouge, M.: Treatment of hepatitis C virus genotype 3-infection. Liver Int. 34(Suppl 1), 18–23 (2014)
European Medicines Agency. Daklinza authorizations details. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003768/human_med_001792.jsp&mid=WC0b01ac058001d124
Dugum, M., O’Shea, R.: Hepatitis C virus: here comes all-oral treatment. Cleve. Clin. J. Med. 81(3), 159–172 (2014)
Leroy, V., Angus, P., Bronowicki, J.P., Dore, G.J., Hezode, C., Pianko, S., Pol, S., Stuart, K., Tse, E., McPhee, F., Bhore, R., Jimenez-Exposito, M.J., Thompson, A.J.: Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+). Hepatology. 63(5), 1430–1441 (2016)
Foster, G.R., Pianko, S., Brown, A., Forton, D., Nahass, R.G., George, J., Barnes, E., Brainard, D.M., Massetto, B., Lin, M., Han, B., McHutchison, J.G., Subramanian, G.M., Cooper, C., Agarwal, K., BOSON Study Group: Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 149(6), 1462–1470 (2015)
McEwan, P., Ward, T., Bennett, H., Kalsekar, A., Webster, S., Brenner, M., Yuan, Y.: Estimating the clinical and economic benefit associated with incremental improvements in sustained virologic response in chronic hepatitis C. PLoS One 10(1), e0117334 (2015)
McEwan, P., Ward, T., Chen, C.J., Lee, M.H., Yang, H.I., Kim, R., L’Italien, G., Yuan, Y.: Estimating the incidence and prevalence of chronic hepatitis C infection in Taiwan using back projection. Value Health Reg. Issues 3, 5–11 (2014)
McEwan, P., Kim, R., Yuan, Y.: Assessing the cost utility of response-guided therapy in patients with chronic hepatitis C genotype 1 in the UK using the MONARCH model. Appl. Health Econ. Health Policy 11(1), 53–63 (2013)
McEwan, P., Ward, T., Yuan, Y., Kim, R., L’italien, G.: The impact of timing and prioritization on the cost-effectiveness of birth cohort testing and treatment for hepatitis C virus in the United States. Hepatology 58(1), 54–64 (2013)
Italian Medicines Agency (AIFA). Pubblicato il nuovo Algoritmo per la terapia dell’Epatite C cronica. Available at: http://www.agenziafarmaco.gov.it/it/content/pubblicato-il-nuovo-algoritmo-la-terapia-dell%E2%80%99epatite-c-cronica
Fattovich, G., Giustina, G., Degos, F., Tremolada, F., Diodati, G., Almasio, P., Nevens, F., Solinas, A., Mura, D., Brouwer, J.T., Thomas, H., Njapoum, C., Casarin, C., Bonetti, P., Fuschi, P., Basho, J., Tocco, A., Bhalla, A., Galassini, R., Noventa, F., Schalm, S.W., Realdi, G.: Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 112(2), 463–472 (1997)
Grieve, R., Roberts, J., Wright, M., Sweeting, M., DeAngelis, D., Rosenberg, W., Bassendine, M., Main, J., Thomas, H.: Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 55(9), 1332–1338 (2006)
Grieve, R., Roberts, J.: Economic evaluation for hepatitis C. Acta Gastroenterol. Belg. 65, 104–109 (2002)
Shepherd, J., Jones, J., Hartwell, D., Davidson, P., Price, A., Waugh, N.: Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol. Assess. 11(11), 1–205, iii (2007)
Bennett, W.G., Inoue, Y., Beck, J.R., Wong, J.B., Pauker, S.G., Davis, G.L.: Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann. Intern. Med. 127(10), 855–865 (1997)
Martin, N.K., Vickerman, P., Miners, A., Foster, G.R., Hutchinson, S.J., Goldberg, D.J., Hickman, M.: Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 55(1), 49–57 (2012)
International Monetary Fund. World Economic Outlook Database, April 2014. Available at: http://www.imf.org/external/pubs/ft/weo/2015/01/weodata/index.aspx
Official Gazette of the Italian Medicines Agency (AIFA). Ex-factory price net of GVT measures as AIFA determination of 03 Jul 2006 and 27 Sept 2006 (2014)
Gruppo multidisciplinare sui farmaci per l’epatite C cronica. Nuovi antivirali diretti nella terapia dell’epatite C cronica. Documento di indirizzo per la definizione delle strategie terapeutiche da applicare sul breve termine. Direzione Generale Cura della Persona, Salute e Welfare - Regione Emilia-Romagna. Aggiornamento Giugno 2016. Available at: http://salute.regione.emilia-romagna.it/documentazione/ptr/elaborati/229-epatite-c-cronica-giugno-2016
Mennini, F.S., Marcellusi, A., Andreoni, M., Gasbarrini, A., Salomone, S., Craxì, A.: Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy. Clin. Outcomes Res. 19(6), 303–310 (2014)
Wright, M., Grieve, R., Roberts, J., Main, J., Thomas, H.C., UK Mild Hepatitis C Trial Investigators: Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol. Assess. 10(21), 1–113, iii (2006)
Castelnuovo, E., Thompson-Coon, J., Pitt, M., Cramp, M., Siebert, U., Price, A., Stein, K.: The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technol. Assess. 10(32), 1–93, iii-iv, ix-xii (2006)
Sutton, A.J., Edmunds, W.J., Sweeting, M.J., Gill, O.N.: The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. J. Viral Hepat. 15(11), 797–808 (2008)
Italian Health Economics Association (AIES): Proposta di Linee-Guida per la valutazione economica degli interventi sanitari. Politiche Sanitarie 10(2), 91–99 (2009)
Russo, P.: La valutazione farmacoeconomica nel contesto regolatorio italiano. Analisi quali-quantitativa dei dossier di richiesta del prezzo e della rimborsabilità. (Pharmacoeconomic evaluations in the Italian regulatory context: a quali-quantitative analysis of pricing and reimbursement dossiers). Pharmacoecon. Ital. Res. Artic. 10, 59–75 (2008)
European Association for Study of Liver: EASL recommendations on treatment of hepatitis C 2015. J. Hepatol. 63(1), 199–236 (2015)
Najafzadeh, M., Andersson, K., Shrank, W.H., Krumme, A.A., Matlin, O.S., Brennan, T., Avorn, J., Choudhry, N.K.: Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann. Intern. Med. 162(6), 407–419 (2015)
Moshyk, A., Martel, M.J., Tahami Monfared, A.A., Goeree, R.: Cost-effectiveness of daclatasvir plus sofosbuvir-based regimen for treatment of hepatitis C virus genotype 3 infection in Canada. J Med Econ. 11, 1–12 (2015)
McEwan, P., Ward, T., Webster, S., Yuan, Y., Kalsekar, A., Kamae, I., Kobayashi, M., Tang, A., Kumada, H.: Estimating the cost-effectiveness of daclatasvir plus asunaprevir in difficult to treat Japanese patients chronically infected with hepatitis C genotype 1b. Hepatol Res. 46(5), 423–433 (2015)
Gimeno-Ballester, V., Mar, J., San, Miguel R.: Cost-effectiveness analysis of simeprevir with daclatasvir for non-cirrhotic genotype-1b-naïve patients plus chronic hepatitis C. Expert Rev. Pharmacoecon. Outcomes Res. 1, 1–10 (2015)
McEwan, P., Bennett, H., Ward, T., Webster, S., Gordon, J., Kalsekar, A., Yuan, Y., Brenner, M.: The cost-effectiveness of daclatasvir-based regimens for the treatment of hepatitis C virus genotypes 1 and 4 in the UK. Eur. J. Gastroenterol. Hepatol. (2015)
Vargas, C.L., Espinoza, M.A., Giglio, A., Soza, A.: Cost effectiveness of daclatasvir/asunaprevir versus peginterferon/ribavirin and protease inhibitors for the treatment of hepatitis C genotype 1b naïve patients in Chile. PLoS One 10(11), e0141660 (2015)
Acknowledgements
This study was supported by Bristol-Myers Squibb. Professional medical writing and editorial assistance was provided by A.L. and A.A., and was funded by Bristol-Myers Squibb. U.R. declares speaker fees (Janssen Cilag). A.A. declares research grants (Abbvie, Gilead, Janssen, MSD), advisory board fees: (Abbvie, Gilead, Janssen, MSD, BMS), and speaker fees (Abbvie, Gilead, Janssen, BMS). A.L. declares advisory board honorarium and speaker fees (BMS, Abbvie, ViiV, Gilead, Janssen Cilag). M.B. declares no competing interests. C.N. is employed by Bristol Myers Squibb S.r.l. D.C. declares advisory board fees (MSD, Abbvie).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Restelli, U., Alberti, A., Lazzarin, A. et al. Cost-effectiveness analysis of the use of daclatasvir + sofosbuvir + ribavirin (16 weeks and 12 weeks) vs sofosbuvir + ribavirin (16 weeks and 24 weeks) for the treatment of cirrhotic patients affected with hepatitis C virus genotype 3 in Italy. Eur J Health Econ 19, 37–44 (2018). https://doi.org/10.1007/s10198-016-0865-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-016-0865-3