Abstract
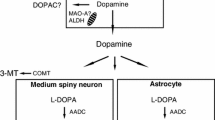
l-Deprenyl is a complex drug, and number of mechanisms have been proposed to explain its effects. These include blockade of dopamine metabolism, amplification of dopamine responses, induction of superoxide dismutase or delaying apoptosis. Usingin situ hybridization techniques, we have shown thatl-deprenyl (5–10 mg/kg intraperitoneally, killed after 24 h) increases aromaticl-amino acid decarboxylase (AADC) mRNA levels in rat substantia nigraventral tegmental area. In human brain tissue, AADC is present at low levels, suggesting a possible rate-limiting role in monoamine synthesis. This is particularly important in parkinsonian patients, since the therapeutic efficacy ofl-DOPA is attributed to its enzymatic decarboxylation to dopamine. The present findings support that one of the effects ofl-deprenyl may be to facilitate the decarboxylation ofl-DOPA by increasing the availability of AADC.
Similar content being viewed by others
Abbreviations
- l-DOPA:
-
3,4-dihydroxyphenylalanine
- MAO B:
-
monoamine oxidase type B
- AADC:
-
aromaticl-amino acid decarboxylase
References
Birkmayer W., Riederer P., Ambrozi and Youdim M. B. (1977) Implications of combined treatment with “Madopar” andl-deprenyl in Parkinson’s disease, a long term study.Lancet 1, 439–443.
Carrillo M., Kanai M., Nokubo M., and Kitani K. (1991) (−)-Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats.Life Sci. 48, 517–521.
Cho S., Duchemin A.-M., Neff N. H., and Hadjiconstantinou M. (1996) Modulation of tyrosine hydroxylase and aromaticl-amino acid decarboxylase after inhibiting monoamine oxidase-A.Eur. J. Pharmacol. 314, 51–59.
Eaton M. J., Gudehithlu K. P., Silvia C. P., Quach T., Hadjiconstantinou M., and Neff N. H. (1993) Distribution of aromatic L-amino acid decarboxylase mRNA in mouse brain by in situ hybridization histology.J. Comp. Neurol. 337, 640–654.
Gibson C. J. (1987) Inhibition of MAO B, but not MAO A, blocks DSP-4 toxicity on central NE neurons.Eur. J. Pharmacol. 141, 135–138.
Glover V., Sandler M., and Owen F. (1977) Dopamine is a monoamine oxidase B substrate in man.Nature 265, 80–81.
Hornykiewicz O. (1966) Dopamine (3-hydroxytyramine) and brain function.Pharmacol. Rev. 18, 925–964.
Jaeger C. B., Rugiero D. A., Albert V. R., Park D. H., Joh T. H., and Reis D. J. (1984) Aromatic L-amino acid decarboxylase in the rat brain: immunocytochemical localization in neurons of the brain stem.Neuroscience 11, 691–713.
Karoum F., Chuang L. W., Eisler T., Calne D. B., Liebowitz M. R., Quintin F. M., et al. (1982) Metabolism of (−)-deprenyl to amphetamine and methamphetamine may be responsible for deprenyl’s therapeutic benefit: A biochemical assessment.Neurology 32, 503–509.
Knoll J. (1988) Extension of life span of rats by long-term (−) deprenyl treatment.Mt. Sinai J. Med. 55, 67–74.
Kosofsky B. E., Genova L. M., and Hyman S. E. (1994) Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain.J. Comp. Neurol. 351, 27–40.
Li X.-M., Juorio A. V., Paterson I. A., Zhu M.-Y., and Boulton A. A. (1992) Specific irreversible MAO B inhibitors stimulate gene expression of aromaticl-amino acid decarboxylase in PC12 cells.J. Neurochem. 59, 2324–2327.
Li X.-M., Jourio A. V., and Boulton A. A. (1993a) NSD-1015 alters the gene expression aromaticl-amino acid decarboxylase in PC12 pheochromocytoma cells.Neurochem. Res. 18, 915–919.
Li X.-M., Juorio A. V., Paterson I. A., Walz W., Zhu M.-Y., and Boulton A. A. (1993b) Gene expression of aromaticl-amino acid decarboxylase in cultured rat glial cells.J. Neurochem. 59, 1172–1175.
Li X.-M., Juorio A. V., and Boulton A. A. (1995) Some new mechanisms underlying the actions of (−)-deprenyl: possible relevance to neurodegeneration.Prog. Brain Res. 106, 99–112.
Lloyd K. G. and Hornykiewicz O. (1970) Parkinson’s disease, activity ofl-dopa decarboxylase in discrete brain regions.Science 170, 1212, 1213.
Marsden C. D. (1990) Parkinson’s disease.Lancet 335, 948–952.
Paterson I. A., Juorio A. V., and Boulton A. A. (1990) 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system?J. Neurochem. 55, 1827–1837.
Paxinos G. and Watson C. (1982)Rat Brain in Stereotaxic Coordinates. Academic, New York.
Robins E., Robins J. M., Croninger A. B., Moses S. G., Spencer J., and Hudgens R. W. (1967) The low level of 5-HTP decarboxylase in human brain.Biochem. Med. 1, 240–251.
Salo P. T. and Tatton W. G. (1992) Deprenyl reduces the death of motoneurons caused by axotomy.J. Neurosci. Res. 31, 394–400.
Tatton W. G. and Chalmers-Redman R. M. E. (1997) Apoptosis in neurogenerative disorders: potential therapy by modifying gene transcription.J. Neural Transm. 49, Suppl, 245–268.
The Parkinson Study Group (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease.N. Engl. J. Med. 328, 176–183.
Youdim B. M. H., Heldman E., Pollard H. B., Fleming P., and McHugh E. (1986) Contrasting monoamine oxidase activity and tyramine induced catecholamine release in PC 12 and chromaffin cells.Neuroscience 19, 1311–1318.
Yu P. H., Davis B. A., Durden D. A., Terleckyj I., Nicklas W. G., and Boulton A. A. (1994) Neurochemical and neuroprotective effects of some aliphatic propargylamines: new selective non-amphetamine-like MAO-B inhibitors.J. Neurochem. 62, 697–704.
Zsilla G., Földi P., Held G., Székely A. M., and Knoll J. (1986) The effect of repeated doses of repeated doses of (−) deprenyl on the dynamics of monoaminergic transmission. Comparison with clorgyline.Pol. J. Pharmacol. Pharm. 38, 57–67.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, XM., Juorio, A.V., Qi, J. et al. l-Deprenyl induces aromaticl-amino acid decarboxylase (AADC) mRNA in the rat substantia nigra and ventral tegmentum. Molecular and Chemical Neuropathology 35, 149–155 (1998). https://doi.org/10.1007/BF02815121
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02815121