Abstract
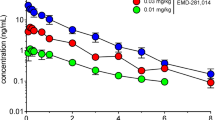
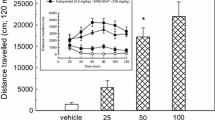
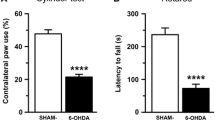
The most effective treatment of Parkinson’s disease (PD) l-DOPA is associated with major side effects, in particular l-DOPA-induced dyskinesia, which motivates development of new treatment strategies. We have previously shown that chronic treatment with a substantially lower dose of deuterium-substituted l-DOPA (D3-l-DOPA), compared with l-DOPA, produced equal anti-parkinsonian effect and reduced dyskinesia in 6-OHDA-lesioned rats. The advantageous effects of D3-l-DOPA are in all probability related to a reduced metabolism of deuterium dopamine by the enzyme monoamine oxidase (MAO). Therefore, a comparative neurochemical analysis was here performed studying the effects of D3-l-DOPA and l-DOPA on dopamine output and metabolism in 6-OHDA-lesioned animals using in vivo microdialysis. The effects produced by D3-l-DOPA and l-DOPA alone were additionally compared with those elicited when the drugs were combined with the MAO-B inhibitor selegiline, used in PD treatment. The different treatment combinations were first evaluated for motor activation; here the increased potency of D3-l-DOPA, as compared to that of l-DOPA, was confirmed and shown to be of equal magnitude as the effect produced by the combination of selegiline/l-DOPA. The extracellular levels of dopamine were also increased following both D3-l-DOPA and selegiline/l-DOPA administration compared with l-DOPA administration. The enhanced behavioral and neurochemical effects produced by D3-l-DOPA and the combination of selegiline/l-DOPA are attributed to decreased metabolism of released dopamine by MAO-B. The similar effect produced by D3-l-DOPA and selegiline/l-DOPA, respectively, is of considerable clinical interest since D3-l-DOPA, previously shown to exhibit a wider therapeutic window, in addition may reduce the need for adjuvant MAO-B inhibitor treatment.





Similar content being viewed by others
Abbreviations
- D3-l-DOPA:
-
Deuterium-substituted l-DOPA
References
Arai R, Karasawa N, Geffard M, Nagatsu I (1995) l-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett 195:195–198
Arai R, Karasawa N, Kurokawa K, Kanai H, Horiike K, Ito A (2002) Differential subcellular location of mitochondria in rat serotonergic neurons depends on the presence and the absence of monoamine oxidase type B. Neuroscience 114:825–835
Azzaro AJ, Demarest KT (1982) Inhibitory effects of type A and type B monoamine oxidase inhibitors on synaptosomal accumulation of [3H]dopamine: a reflection of antidepressant potency. Biochem Pharmacol 31:2195–2197
Barnum CJ, Bhide N, Lindenbach D, Surrena MA, Goldenberg AA, Tignor S, Klioueva A, Walters H, Bishop C (2012) Effects of noradrenergic denervation on l-DOPA-induced dyskinesia and its treatment by alpha- and beta-adrenergic receptor antagonists in hemiparkinsonian rats. Pharmacol Biochem Behav 100:607–615
Bartl J, Müller T, Grünblatt E, Gerlach M, Riederer P (2014) Chronic monoamine oxidase-B inhibitor treatment blocks monoamine oxidase-A enzyme activity. J Neural Transm 121:379–383
Brown EE, Damsma G, Cumming P, Fibiger HC (1991) Interstitial 3-methoxytyramine reflects striatal dopamine release: an in vivo microdialysis study. J Neurochem 57:701–707
Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW (1990) Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J Neurochem 55:981–988
Cenci MA, Whishaw IQ, Schallert T (2002) Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci 3:574–579
Colzi A, D’Agostini F, Cesura AM, Borroni E, Da Prada M (1993) Monoamine oxidase-A inhibitors and dopamine metabolism in rat caudatus: evidence that an increased cytosolic level of dopamine displaces reversible monoamine oxidase-A inhibitors in vivo. J Pharmacol Exp Ther 265:103–111
Di Chiara G, Tanda G, Carboni E (1996) Estimation of in vivo neurotransmitter release by brain microdialysis: the issue of validity. Behav Pharmacol 7:640–657
Encarnacion EV, Hauser RA (2008) Levodopa-induced dyskinesias in Parkinson’s disease: etiology, impact on quality of life, and treatments. Eur Neurol 60:57–66
Fagervall I, Ross SB (1986) A and B forms of monoamine oxidase within the monoaminergic neurons of the rat brain. J Neurochem 47:569–576
Finberg JP, Wang J, Goldstein DS, Kopin IJ, Bankiewicz KS (1995) Influence of selective inhibition of monoamine oxidase A or B on striatal metabolism of l-DOPA in hemiparkinsonian rats. J Neurochem 65:1213–1220
Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F (2007) Abnormal involuntary movements (AIMs) following pulsatile dopaminergic stimulation: severe deterioration and morphological correlates following the loss of locus coeruleus neurons. Brain Res 1135:219–229
Hefti F, Melamed E, Wurtman RJ (1981) The site of dopamine formation in rat striatum after l-dopa administration. J Pharmacol Exp Ther 217:189–197
Heinonen EH, Rinne UK (1989) Selegiline in the treatment of Parkinson’s disease. Acta Neurol Scand Suppl 126:103–111
Ives NJ, Stowe RL, Marro J, Counsell C, Macleod A, Clarke CE, Gray R, Wheatley K (2004) Monoamine oxidase type B inhibitors in early Parkinson’s disease: meta-analysis of 17 randomised trials involving 3525 patients. BMJ 329:593
Jahng JW, Houpt TA, Wessel TC, Chen K, Shih JC, Joh TH (1997) Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse 25:30–36
Kannari K, Tanaka H, Maeda T, Tomiyama M, Suda T, Matsunaga M (2000) Reserpine pretreatment prevents increases in extracellular striatal dopamine following l-DOPA administration in rats with nigrostriatal denervation. J Neurochem 74:263–269
Kannari K, Shen H, Arai A, Tomiyama M, Baba M (2006) Reuptake of l-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci Lett 402:62–65
Karhunen T, Tilgmann C, Ulmanen I, Panula P (1995) Catechol-O-methyltransferase (COMT) in rat brain: immunoelectron microscopic study with an antiserum against rat recombinant COMT protein. Neurosci Lett 187:57–60
Karoum F, Chuang LW, Eisler T, Calne DB, Liebowitz MR, Quitkin FM, Klein DF, Wyatt RJ (1982) Metabolism of (-) deprenyl to amphetamine and methamphetamine may be responsible for deprenyl’s therapeutic benefit: a biochemical assessment. Neurology 32:503–509
Kastner A, Anglade P, Bounaix C, Damier P, Javoy-Agid F, Bromet N, Agid Y, Hirsch EC (1994) Immunohistochemical study of catechol-O-methyltransferase in the human mesostriatal system. Neuroscience 62:449–457
Kato T, Dong B, Ishii K, Kinemuchi H (1986) Brain dialysis: in vivo metabolism of dopamine and serotonin by monoamine oxidase A but not B in the striatum of unrestrained rats. J Neurochem 46:1277–1282
Kuczenski R, Segal DS (1992) Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J Pharmacol Exp Ther 262:1085–1094
Kushner DJ, Baker A, Dunstall TG (1999) Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol 77:79–88
Lai JC, Leung TK, Guest JF, Lim L, Davison AN (1980) The monoamine oxidase inhibitors clorgyline and L-deprenyl also affect the uptake of dopamine, noradrenaline and serotonin by rat brain synaptosomal preparations. Biochem Pharmacol 29:2763–2767
Lamensdorf I, Youdim MB, Finberg JP (1996) Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum in vivo. J Neurochem 67:1532–1539
Larsen JP, Boas J, Erdal JE (1999) Does selegiline modify the progression of early Parkinson’s disease? Results from a five-year study. The Norwegian-Danish Study Group. Eur J Neurol 6:539–547
Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
Li XM, Juorio AV, Paterson IA, Walz W, Zhu MY, Boulton AA (1992) Gene expression of aromatic L-amino acid decarboxylase in cultured rat glial cells. J Neurochem 59:1172–1175
Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA (2010) l-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1465–1476
Lopez-Real A, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL (2003) Localization and functional significance of striatal neurons immunoreactive to aromatic L-amino acid decarboxylase or tyrosine hydroxylase in rat Parkinsonian models. Brain Res 969:135–146
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Malmlöf T, Svensson TH, Schilström B (2008) Altered behavioural and neurochemical profile of l-DOPA following deuterium substitutions in the molecule. Exp Neurol 212:538–542
Malmlöf T, Rylander D, Alken RG, Schneider F, Svensson TH, Cenci MA, Schilström B (2010) Deuterium substitutions in the l-DOPA molecule improve its anti-akinetic potency without increasing dyskinesias. Exp Neurol 225:408–415
Melamed E, Hefti F, Pettibone DJ, Liebman J, Wurtman RJ (1981) Aromatic L-amino acid decarboxylase in rat corpus striatum: implications for action of l-dopa in parkinsonism. Neurology 31:651–655
Mercuri NB, Bernardi G (2005) The ‘magic’ of l-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci 26:341–344
Miller DW, Abercrombie ED (1999) Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous l-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem 72:1516–1522
Mura A, Jackson D, Manley MS, Young SJ, Groves PM (1995) Aromatic L-amino acid decarboxylase immunoreactive cells in the rat striatum: a possible site for the conversion of exogenous l-DOPA to dopamine. Brain Res 704:51–60
Mura A, Linder JC, Young SJ, Groves PM (2000) Striatal cells containing aromatic L-amino acid decarboxylase: an immunohistochemical comparison with other classes of striatal neurons. Neuroscience 98:501–511
Nakamura K, Ahmed M, Barr E, Leiden JM, Kang UJ (2000) The localization and functional contribution of striatal aromatic L-amino acid decarboxylase to l-3,4-dihydroxyphenylalanine decarboxylation in rodent parkinsonian models. Cell Transplant 9:567–576
Navailles S, Bioulac B, Gross C, De Deurwaerdere P (2010) Serotonergic neurons mediate ectopic release of dopamine induced by l-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis 38:136–143
Nevalainen N, Af Bjerken S, Lundblad M, Gerhardt GA, Strömberg I (2011) Dopamine release from serotonergic nerve fibers is reduced in l-DOPA-induced dyskinesia. J Neurochem 118:12–23
Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM (1997) Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse 26:194–198
Nutt JG (2008) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23(Suppl 3):S580–S584
Pålhagen S, Heinonen E, Hägglund J, Kaugesaar T, Maki-Ikola O, Palm R (2006) Selegiline slows the progression of the symptoms of Parkinson disease. Neurology 66:1200–1206
Paterson IA, Juorio AV, Berry MD, Zhu MY (1991) Inhibition of monoamine oxidase-B by (-)-deprenyl potentiates neuronal responses to dopamine agonists but does not inhibit dopamine catabolism in the rat striatum. J Pharmacol Exp Ther 258:1019–1026
Paxinos G, Watson C (2008) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Reynolds GP, Elsworth JD, Blau K, Sandler M, Lees AJ, Stern GM (1978) Deprenyl is metabolized to methamphetamine and amphetamine in man. Br J Clin Pharmacol 6:542–544
Riederer P, Youdim MB (1986) Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem 46:1359–1365
Sader-Mazbar O, Loboda Y, Rabey MJ, Finberg JPM (2013) Increased l-dopa-derived dopamine following selective MAO-A or -B inhibition in rat striatum depleted of dopaminergic and serotonergic innervations. Br J Pharmacol 170:999–1013
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123(Pt 11):2297–2305
Schwarting RK, Huston JP (1996) The unilateral 6-hydroxyopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50:275–331
Sharma JC, Macnamara L, Hasoon M, Vassallo M, Ross I (2006) Cascade of levodopa dose and weight-related dyskinesia in Parkinson’s disease (LD-WD-PD cascade). Parkinsonism Relat Disord 12:499–505
Sharma JC, Ross IN, Rascol O, Brooks D (2008) Relationship between weight, levodopa and dyskinesia: the significance of levodopa dose per kilogram body weight. Eur J Neurol 15:493–496
Stanley N, Salem A, Irvine RJ (2007) The effects of co-administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) or para-methoxyamphetamine and moclobemide at elevated ambient temperatures on striatal 5-HT, body temperature and behavior in rats. Neuroscience 146:321–329
Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M (1999) Role of serotonergic neurons in l-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport 10:631–634
Tashiro Y, Kaneko T, Sugimoto T, Nagatsu I, Kikuchi H, Mizuno N (1989) Striatal neurons with aromatic L-amino acid decarboxylase-like immunoreactivity in the rat. Neurosci Lett 100:29–34
Ungerstedt U (1971) Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 367:69–93
Ungerstedt U (1976) 6-hydroxydopamine-inuced degeneration of the dopaminergic pathway: the turning syndrome. Pharmacol Ther B 2:37–40
Wachtel SR, Abercrombie ED (1994) L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J Neurochem 63:108–117
Waldmeier PC (1987) Amine oxidases and their endogenous substrates (with special reference to monoamine oxidase and the brain). J Neural Transm Suppl 23:55–72
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 25:439–456
Yamada H, Aimi Y, Nagatsu I, Taki K, Kud M, Arai R (2007) Immunohistochemical detection of l-DOPA-derived dopamine within serotonergic fibers in the striatum and the substantia nigra pars reticulata in Parkinsonian model rats. Neurosci Res 59:1–7
Yu PH, Bailey BA, Durden DA, Boulton AA (1986) Stereospecific deuterium substitution at the alpha-carbon position of dopamine and its effect on oxidative deamination catalyzed by MAO-A and MAO-B from different tissues. Biochem Pharmacol 35:1027–1036
Acknowledgements
We thank Mrs. Anna Malmerfelt for excellent technical assistance. This study was supported by the Swedish Research Council (Grant No. 4747), the Swedish Parkinson´s disease Association, the Swedish Brain Foundation, the Lars Hierta Foundation and Karolinska Institutet. BiRDS Pharma GmbH, has partly supported this study by a research grant to the Karolinska Institutet.
Conflict of interest
RG. Alken and F. Schneider are both scientists supported by BiRDs Pharma GmbH (part of the BDD/CDRD Group), the manufacturers of D3-l-DOPA. T H. Svensson has received research grants from AstraZeneca, Organon, Schering-Plough, Merck and Johnson & Johnson and has served at AstraZeneca and Lundbeck scientific advisory board meetings. T. Malmlöf, K. Feltmann, Å. Konradsson-Geuken and B. Schilström declare that, except for income received from our universities, no financial support or compensation has been received over the past 3 years for research or professional service that within this context could be considered as constituting a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malmlöf, T., Feltmann, K., Konradsson-Geuken, Å. et al. Deuterium-substituted l-DOPA displays increased behavioral potency and dopamine output in an animal model of Parkinson’s disease: comparison with the effects produced by l-DOPA and an MAO-B inhibitor. J Neural Transm 122, 259–272 (2015). https://doi.org/10.1007/s00702-014-1247-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1247-6