Abstract
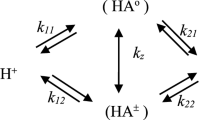
We have used a potentiometric method to determine the thermodynamic equilibrium constants for the macroscopic ionization processes of 5-deoxypyridoxal (DPL) in water-dioxane mixtures (0-70% weight fraction in dioxane) at temperatures ranging from 10°C to 50°C. These data, together with previously published equilibrium constants for the tautomerism and hydration processes, have allowed us to resolve the complete microconstant system. We have also calculated the microscopic ionization equilibrium constants under all the experimental conditions. The changes of standard thermodynamic function for the macroscopic and microscopic ionization processes were obtained in various water-dioxane mixtures at 25°C. The values of a given microscopic pK with different solvents and temperatures fit very well to an equation which relates this magnitude with the thermodynamic parameters, the solvation of the components of the reaction, and a solvent parameter. We have obtained an interesting linear correlation between the thermodynamic parameters corresponding to all the microscopic ionizations of DPL and the net change of the solvation during the process: enthalpies correlate linearly for all the microscopic ionizations, while entropies do so for the phenols and pyridinium ions separately.
Similar content being viewed by others
References
P. Christen and D. E. Metzler,Transaminases, (Wiley, New York, 1985).
E. E. Snell, inVitamin B 6 Pyridoxal Phosphate: Chemical, Biochemical and Medical Aspects, Part A, D. Dolphin, R. Poulson and O. Abramovic, eds, (Wiley, New York, 1986).
M. Cortijo, J. Llor, and J. M. Sánchez-Ruiz,J. Biol. Chem. 263, 17960 (1988).
A. E. Martell and R. J. Motekaitis,The Determination and Use of Stability Constants, Chap. 7, (VCH, New York, 1988).
J. Polster and H. Lachmann,Spectrometric Titrations, Chap. 12, (VCH, New York, 1989).
J. Llor, O. Lopez-Mayorga, and L. Muñoz,Magn. Reson. Chem. 31, 552 (1993).
J. Llor and S. B. Asensio,J. Solution Chem. 25, 667 (1996).
C. Iwata,Biochem. Prep. 12, 117 (1968).
J. M. Sanchez-Ruiz, J. Llor, and M. Cortijo,J. Chem. Soc. Perkin Trans. II 2047 (1984).
E. A. Peterson and H. A. Sober,J. Am. Chem. Soc. 76, 169 (1954).
L. G. van Uitert and C. E. Haas,J. Am. Chem. Soc. 75, 451 (1953).
L. G. van Uitert and W. C. Femelius,J. Am. Chem. Soc. 76, 5887 (1954).
J. Llor, M. Sánchez-Nevado, S. Asensio, and M. Cortijo,An. Quim. 83, 317 (1987).
E. M. Woolley, D. G. Hurkot, and L. H. Hepler,J. Phys. Chem. 74, 3908 (1970).
C. C. Panichajakul and E. M. Woolley,Anal. Chem. 47, 1870 (1975).
C. C. Panichajakul and E. M. Woolley,Adv. Chem. Ser. 155, 263 (1976).
J. Llor, J. M. Sanchez-Ruiz, and M. Cortijo,Acta Cient. Comp. 22, 231 (1985).
S. B. Asensio, E. Lopez-Cantarero, and J. Llor,Can. J. Chem. 70, 1635 (1992).
C. M. Harris, R. J. Johnson, and D. E. Metzler,Biochim. Biophys. Acta 421, 181 (1976).
D. E. Metzler and E. E. Snell,J. Am. Chem. Soc. 77, 2431 (1955).
D. D. Perrin, B. Dempsey, and E. P. Sarjeant. pKa Prediction for Organic Acid and Bases. Chapman and Hall, London (1981).
W. L. Marshall and A. S. Quist,Proc. Natl. Acad. Sci. U.S.A.,58, 901 (1967).
A. S. Quist and W. L. Marshall,J. Phys. Chem. 72, 1536 (1968)
W. L. Marshall,J. Phys. Chem. 74, 346 (1970).
E. C. W. Clarke and D. N. Glew,Trans. Faraday Soc. 62, 539 (1966).
J. Datta and K. K. Kundu,Can J. Chem. 59, 3149 (1981).
N. S. Isaacs.Physical Organic Chemistry. Longman Scientific & Technical, (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Llor, J., Ros, M.P. & Asensio, S.B. Complete resolution of the ionization equilibria of 5-deoxypyridoxal in water-dioxane mixtures. J Solution Chem 26, 1021–1036 (1997). https://doi.org/10.1007/BF02768827
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02768827