Abstract
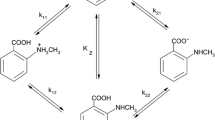
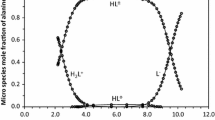
Knowledge of the protonation constants of α-amino acids is important, interesting and necessary for complete understanding of the physiochemical behavior of the acids. The acid–base chemistry of zwitterionic compounds such as α-amino acids has been characterized in terms of the macroscopic constants; K1, K2; the microscopic constants; k11, k12, k21, k22 and the tautomeric constant; kz. The compounds may exist in four different microforms; a cation, zwitterion (dipolarion), neutral species and anion. In this study, both to demonstrate whether the predominant species are zwitterion or the neutral form and to predict the change of dipolar form to neutral form ratio in dioxane–water mixtures, the macroscopic protonation constants of the α-amino acids, glycine, DL-alanine, DL-valine, L-leucine, DL-phenylalanine and L-serine, and their esters, were determined potentiometrically using a combined pH electrode system calibrated as the concentration of hydrogen ion and later the microscopic and tautomeric constants of the amino acids were calculated in dioxane–water mixtures (20, 40, and 60% dioxane (v/v)). Titrations were performed at 25.0 ± 0.1 °C and the ionic strength of the medium was maintained at 0.10 mol·dm−3 using sodium chloride. The protonation constants were influenced by changes in solvent composition and the effects of solvent composition on the protonation constants are discussed. Also, the variation of microscopic constants and the ratio of zwitterionic form to neutral form are discussed on the basis of solute–solvent interactions of amino acids in dioxane–water mixtures.

Similar content being viewed by others
References
Dogan, A., Demirel, A., Kılıç, E.: The protonation equilibria of selected glycine dipeptides in ethanol–water mixture: solvent composition effect. Amino Acids 36, 373–379 (2009)
Albert, A., Serjeant, E.P.: The Determination of Ionization Constants. Chapman & Hall, New York (1984)
Pandit, N.K., Sisco, J.M.: The determination of microscopic ionization constants of a substituted piperazine using estimates from model compounds. Pharm. Res. 6(2), 177–181 (1989)
Szakacs, Z., Krazni, M., Noszal, B.: Determination of microscopic acid–base parameters from NMR–pH titrations. Anal. Bioanal. Chem. 378, 1428–1448 (2004)
Tager, H.S.: Solute translocations: an overview of biological transport. In: Colombett, L.G. (ed.) Biological Transport of Radionucleides. CRC Press, Boca Raton (1982)
Stein, W.D., Lieb, W.R.: Transport and Diffusion Across Cell Membranes. Academic Press, Orlando (1986)
Hughes, A.B.: Amino Acids, Peptides and Proteins in Organic Chemistry, Analysis and Function of Amino Acids and Peptides. Wiley-VCH, Hoboken (2012)
Nikaido, H., Thanassi, D.G.: Penetration of lipophilic agents with multiple protonation sites into bacterial cells. Antimicrob. Agents Chem. 37, 1393–1399 (1993)
Martin, R.B., Edsall, J.T., Wetlaufer, D.B., Hollingworth, B.R.: A complete ionization scheme for tyrosine, and the ionization constants of some tyrosine derivatives. J. Biol. Chem. 233, 1429–1435 (1958)
Hughes, D.L., Bergan, J., Grabowski, E.J.: Amino acid chemistry in dipolar aprotic solvents; dissociation constants and ambident reactivity. J. Org. Chem. 51, 2579–2585 (1986)
D’Angelo, J.C., Collette, T.W.: A method for the measurement of site-specific tautomeric and zwitterionic microspecies equilibrium constants. Anal. Chem. 69, 1642–1650 (1997)
Martin, B.: Zwitterions formation upon deprotonation in L-3,4-dihidroxyphenylalanine and other phenolic amines. J. Phys. Chem. 75, 2657–2661 (1971)
Novak, T., Kökösi, J., Podanyi, B., Nozsal, B., Tsai, R.S., Lisa, G., Corrupt, P.A., Testa, B.: Microscopic protonation/deprotanation equilibria of the anti-inflammatory agent piroxicam. Helv. Chim. Acta 78, 553–563 (1995)
Noszal, B., Szaka, Z.: Microscopic protonation equilibria of oxidized glutathione. J. Phys. Chem. B 107, 5074–5080 (2003)
Zapała, L., Woznicka, E., Kalembkiewicz, J.: Tautomeric and microscopic protonation equilibria of anthranilic acid and its derivatives. J. Solution Chem. 43, 1167–1183 (2014)
Carty, R.P., Hirs, C.H.W.: Modification of bovine pancreatic ribonuclease A with 4-sulfonyloxy-2-nitrofluorobenzene. Isolation and identification of modified proteins. J. Biol. Chem. 243, 5256–5265 (1968)
Schmidt, D.E., Westheimer, F.H.: pK of the lysine amino group at the active site of acetoacetate decarboxylase. Biochemistry 10, 249–1253 (1971)
Crosby, J., Stone, R., Lienhard, G.E.: Mechanisms of thiamine-catalyzed reactions. Decarboxylation of 2-(1-carboxy-1-hydroxyetyl)-3,4-dimethylthiazolium chloride. J. Am. Chem. Soc. 92, 2891–2900 (1970)
Bester-Rogac, M., Neueder, R., Barthel, J.: Conductivity of sodium chloride in water +1,4-dioxane mixtures at temperatures from 5 to 35 °C – I. Dilute solutions. J. Solution Chem. 28, 1071–1086 (1999)
Perrin, D.D., Armerega, W.L.F.: Purification of Laboratory Chemicals. Pergamon Press, Elmsford (1991)
Gran, G.: Determination of the equivalent point in potentiometric titrations Part II. Analyst 77, 661–671 (1952)
Martell, A.E., Motekaitis, R.J.: The Determination and Use of Stability Constants. VCH, Weinheim (1988)
Meloun, M., Havel, J., Högfelt, H.: Computation of Solution Equilibria. Wiley, New York (1988)
Woolley, E.M., Hukot, D.G., Hepler, L.G.: Ionization constant for water in aqueous organics mixture. J. Phys. Chem. 74, 3908–3913 (1970)
Rondinini, S., Mussini, T., Mussini, P.R., Longhi, P.: Autoprotolysis constants in nonaqueous solvent and aqueous organic-solvent mixtures. Pure Appl. Chem. 59, 1693–1702 (1987)
Motekaitis, R.J., Martell, A.E.: BEST-a new program for rigorous calculation of equilibrium parameters of complex multi-component systems. Can. J. Chem. 60, 2403–2409 (1982)
Doğan, A., Aslan, N., Canel, E., Kılıç, E.: Solvent effect on the protonation constants of some amino acid esters in 1,4-dioxan–water mixtures. J. Solution Chem. 39(11), 1589–1596 (2010)
Köseoğlu, F., Kılıç, E., Doğan, A.: Studies on the protonation constants and solvation of α-amino acids in dioxan– water mixtures. Anal. Biochem. 277, 243–246 (2000)
Pagliara, A., Carrupt, P.A., Caron, G., Gaillard, P., Testa, B.: Lipophilicity profiles of ampholytes. Chem. Rev. 97, 3385–3400 (1997)
Metzler, D.E., Snell, E.E.: Spectra and ionization constants of the vitamin B6 group and related 3-hydroxypyridine derivatives. J. Am. Chem. Soc. 77, 2431–2437 (1955)
Takas-Novak, K., Avdeef, A., Box, K.J., Podanyi, B., Szasz, G.: Determination of protonation macro and microconstants and octanol/water partition coefficient of the anti-inflammatory drug niflumic acid. J. Pharm. Biomed. Anal. 12, 1369–1377 (1994)
Takas-Novak, K., Tam, K.Y.: Multiwavelength spectrophotometric determination of acid dissociation constants. Part V: Microconstants and tautomeric ratios of diprotic amphoteric drugs. J. Pharm. Biomed. Anal. 21, 1171–1182 (2000)
Mandic, Z., Gabelica, V.: Ionization, lipohpilicity and solubility properties of repaglinide. J. Pharm. Biomed. Anal. 41, 866–871 (2006)
Rossotti, H.: The Study of Ionic Equilibria. Longman, London, New York (1978)
Benesch, R.E., Benesch, R.: The acid strength of the –SH group in cysteine and related compounds. J. Am. Chem. Soc. 77(5), 877–5881 (1955)
Rochester, C.H.: Ionization of phenol, the cresols, and the xylenols in methanol. J. Chem. Soc. Faraday Trans. I 62, 355–358 (1966)
Dogan, A., Aslan, N., Erden, P., Canel, E., Kılıç, E.: Macroscopic and microscopic protonation equilibria of some α-amino acids in dimethyl sulfoxide–water mixtures. J. Solution Chem. 44, 1705–1722 (2015)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions. Academic Press, New York (1959)
Gharib, F., Naeej, N.B., Nouri, N.: Tautomeric and microscopic protonation constants of alanine and valine in different aqueous solutions of methanol and tetrahydrofuran. J. Solution Chem. 44, 1655–1672 (2015)
Headley, A.D., Starnes, S.D.: Effects of branching on the tautomeric equilibrium of amino acids. J. Am. Chem. Soc. 117, 9309–9313 (1995)
Dogan, A., Kılıç, E.: Tautomeric and microscopic protonation equilibria of some α-amino acids. Anal. Biochem. 365, 7–13 (2007)
Gündüz, T., Kılıç, E., Köseoğlu, F., Canel, E.: Protonation constants of some substituted salicylideneanilines in dioxan–water mixtures. Anal. Chim. Acta 282, 489–495 (1993)
Chattopadhyay, A.K., Lahiri, S.C.: Studies on the solvation of amino acids in ethanol and water mixtures. Electrochim. Acta 27, 269–272 (1982)
Kılıç, E., Köseoğlu, F., Başgut, Ö.: Protonation constants of some pyridine derivatives in ethanol–water mixtures. Anal. Chim. Acta. 294, 215–220 (1994)
Kılıç, E., Gökçe, G., Canel, E.: The protonation constants of some aliphatic alkylamines in ethanol–water mixtures. Turk. J. Chem. 26, 843–849 (2002)
Dogan, A., Kılıç, E.: Potentiometric studies on the protonation constants and solvation of some α-amino acid benzyl- and t-butyl- esters in ethanol–water mixtures. Turk. J. Chem. 29, 41–47 (2005)
Takamuku, T., Yamaguchi, A., Tabata, M., Nishi, N., Yoshida, K., Wakita, H., Yamaguchi, T.: Structure and dynamics of 1,4-dioxane–water binary solutions studied by X-ray diffraction, mass spectrometry and NMR relaxation. J. Mol. Liq. 83, 163–177 (1999)
Marcus, Y.: Preferential solvation in mixed solvents X. Completely miscible aqueous co-solvent binary mixtures at 298.15K. Monatsh. Chem. 132, 1387–1411 (2001)
Bates, R.G.: Solute–solvent interactions and acid–base dissociation in mixed solvent systems. J. Electroanal. Chem. 29, 1–19 (1971)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanalp, T.D., Doğan, A. The Microscopic and Tautomeric Protonation Constants of Some α-Amino Acids in Dioxane–Water Mixtures . J Solution Chem 50, 983–994 (2021). https://doi.org/10.1007/s10953-021-01099-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01099-y