Abstract
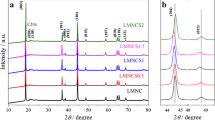
Li0.7[Li1/12Ni1/12Mn5/6]O2 and Li0.7[Li1/12Ni1/12Mn5/6]O2-ySy (y=0.1, 0.2, 0.3) powders were synthesized by using a sol-gel method. As-prepared samples showed typical rhombohedral O3 layered structure. The shape of the initial discharge curve for the samples was almost equal to that of the layered structure. However, the electrode materials were transferred from layered to spinel structures with cycling. At the first cycle, Li0.7[Li1/12Ni1/122Mn25/6]O2 and Li0.7[Li1/12Ni1/12 Mn5/6]O1.9S0.1, Li0.7[Li1/12Ni1/12Mn5/6]O1.8S0.2, and Li0.7[Li1/12Ni1/12Mn5/6]O1.7S0.3 delivered the discharge capacities of 238, 230,224, and 226 mAh/g, respectively, with their capacity fading rates of 0.34, 0.21, 0.12, 0.25%/cycle, respectively. The partial substitutions of Ni and S for Mn and O in Li0.7[Li1/12Ni1/12Mn1/12]O2 significantly enhanced the electrochemical properties of the lithium manganese oxide materials.
Similar content being viewed by others
References
Amatucci, G.G., Pereira, N., Zheng, T. and Tarascon, J.-M.,“Failure Mechanism and Improvement of the Elevated Temperature Cycling of LiMn2O4 Compounds Through the Use of the LiAlxMn2-xO4-zFz Solid Solution,”J. Electrochem. Soc.,148, A171 (2001).
Ammundsen, B. and Paulsen, J.,“Novel Lithium-Ion Cathode Materials Based on Layered Manganese Oxides,”Afo Mater.,13, 943 (2001).
Ammundsen, B., Desilvestro, J., Groutso, T., Hassell, K., Metson, J. B., Regan, E., Steiner, R. and Pickering, P., “Layered LiMn1-xAlyO2 Materials for Lithium Secondary Batteries,”J. Electrochem. Soc.,143, 879 (1996).
Armstrong, A. R., Gitzendanner, R., Robertson, A. D. and Bruce, P. G.,“The Intercalation Compound Li(Mn0.9Co0.1)O2 as a Positive Electrode for Rechargeable Lithium Batteries,”Chem. Commun., 1833 (1998).
Armstrong, A. R., Robertson, A. D., Gitzendanner, R. and Bruce, P. G., “The Layered Intercalation Compounds Li(Mn1-xCoy)O2: Positive Electrode Materials for Lithium-Ion Batteries,”J. Solid State Chem.,145, 549(1999).
Bruce, P. G., Armstrong, A. R. and Gitzendanner, R. L.,“New Intercalation Compounds for Lithium Batteries: Layered LiMnO2,”J. Mater. Chem.,1, 193 (1999).
Chiang, Y.-M., Sadoway, D. R., Jang, Y.-I., Huang, B. and Wang, H., “High Capacity, Temperature-Stable Lithium Aluminum Manganese Oxide Cathodes for Rechargeable Batteries,”Electrochem. Solid-State Lett.,2, 107 (1999).
Dahn, J. R., Scken, U. V. and Michal, C. A.,“Structure and Electrochemistry of Li1±yNiO2 and aNew Li2NiO2 Phase with the Ni(OH)2 Structure,”Solid State Ionics,44, 87 (1990).
Davidson, I. J., McMillan, R J., Slegr, H., Luan, B., Kargina, I., Murray, J. J. and Swainson, I. P., “Electrochemistry and Structure of Li2-xCry Mn2-yO4 Phases,”J. Power Sources,82, 406 (1999).
Goodenough J. B.,Le Journal de Physique et le Radium,20, 155 (1959).
Guyomard, D. and Tarascon, J. M.,“The Carbon/Li1+xMn2O4 System,”Solid State Ionics,69, 222 (1994).
Jang, Y.-I., Huang, B., Chiang, Y.-M. and Sadoway, D. R.,“Stabilization of LiMnO2 in the-NaFeO2 Structure Type by LiAlO2 Addition,”Electrochem. Solid-State Lett.,1, 13 (1998).
Lee, Y. S., Sun, Y. K. and Nahm, K S.,“Synthesis and Characterization of LiNiO2 Cathode Material Prepared by an Adipic Acid-assisted Sol-gel Method for Lithium Secondary Batteries,”Solid State Ionics,118, 159 (1999).
Naghash, A. R. and Lee, J. Y.,“Lithium Nickel Oxyfluoride (Li1-zNi1+z FyO2-y) and Lithium Magnesium Nickel Oxide (Li1-z(MgxNi1-z)1+zO2) Cathodes for Lithium Rechargeable Batteries: II. Electrochemical Investigations,”Electrochim. Acta,46, 2293 (2001).
Naghash, A. R. and Lee, J. Y.,“Lithium Nickel Oxyfluoride (Li1-zNi1+z FyO2-y) and Lithium Magnesium Nickel Oxide (Li1-z(MgxNi1-x)1+zO2) Cathodes for Lithium Rechargeable Batteries: Part I. Synthesis and Characterization of Bulk Phases,”Electrochem. Acta,46, 941 (2001).
Nitta, Y., Okamura, K., Haraguchi, K., Kobayashi, S. and Ohata, A., “Crystal Structure Study of LiNi1-xMnxO2”,J. Power Sources,54, 511 (1995).
Ohuzuku, T., Ueda, A. andNagyama, M.,“Electrochemistry and Structural Chemistry of LiNiO2 (R-3m) for 4 Volt Secondary Lithium Cells,”J. Electrochem. Soc.,140, 1862 (1993).
Park, K S., Cho, M. H., Park, S. H., Nahm, K. S., Sun, Y. K., Lee, Y. S. and Masaki, Y., “The Effects ofNi and Li Doping on the Performance of Lithium Manganese Oxide Material for Lithium Secondary Batteries,”Electrochimica Acta,47, 2937 (2002).
Park, K. S., Cho, M. H., Jin, S. J., Song, C. H. and Nahm, K. S.,“The Effect ofNi Doping on the Performance of O3-Lithium Manganese Oxide Material,”Korean J. Chem. Eng.,5, 21 (2004).
Park, S. H., Park, K. S., Cho, M. H., Sun, Y. K., Nahm, K. S., Lee, Y. S. and Yosio, M., “The Effects of Oxygen Flow Rate and Anion Doping on the Performance of the LiNiO2 Electrochemical for Lithium Secondary Batteries,”Korean J. Chem. Eng.,19, 791 (2002).
Park, S. H., Park, K. S., Moon, S. S., Sun, Y. K. and Nahm, K. S.,“Synthesis and Electrochemical Characterization of Li0.1Mg0.1Mn0.9O3.99 S0.01 using Sol-gel Method,”J. Power Sources,92, 244 (2001).
Park, S. H., Park, K. S., Sun, Y. K. and Nahm, K. S.,“Synthesis and Characterization of a New Spinel, Li,1.02Al0.25Mn1.75O3.97S0.03 Operating at Potentials Between 4.3 and 2.4”V,” J. Electrochem. Soc.,147, 2116(2000).
Park, S. H., Sun, Y. K., Park, K. S., Nahm, K. S., Lee, Y. S. and Masaki, Y., “Synthesis and Electrochemical Properties of Lithium Nickel Oxysulfide (LiNiSyO2-y) Material for Lithium Secondary Batteries,”Electrochim Acta,47, 1721 (2002).
Paulson,J. M. and Dahn, J. R.,“O2-Type Li2/3[Ni1/3Mn2/3]O2: A New Layered Cathode Material for Rechargeable Lithium Batteries II. Structure, Composition, and Properties,”J. Electrochem. Soc.,147, 2478 (2000).
Quine, T. E., Duncan, M. J., Armstrong, A. R., Robertson, A. D. and Bruce, P. G., “Layered LixMn1-yNiyO2 Intercalation Electrodes,”J. Mater. Chem.,12, 2838 (2000).
Sun, Y. K. and Kim, D. W.,“Synthesis and Electrochemical Characterization of LiMn2O4 Cathode Materials for Lithium Polymer Batteries,”KoreanJ. Chem. Eng.,16, 449 (1999).
Sun, Y. K., Kim, D. W., Jin, S. H., Hyung, Y. E. and Moon, S. I.,“Synthesis and Cycling Behavior of LiMn2O4 Cathode materials Prepared byGlycine-assisted Sol-gel Method for Lithium Secondary Batteries,”KoreanJ. Chem. Eng.,15, 64 (1998).
Sun, Y.-K., Lee, Y.-S. and Yoshio, M.,“Cycling Behavior of the Oxysulfide LiAl0.18Mn1.82O3.97S0.03 Cathode Materials at Elevated Temperature,”Materials Letters,56, 4,418 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K.S., Cho, M.H., Jin, S.J. et al. The effects of sulfur doping on the performance of O3-Li0.7[Li1/12Ni1/12Mn5/6]O2 powder. Korean J. Chem. Eng. 22, 560–565 (2005). https://doi.org/10.1007/BF02706643
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706643