Abstract
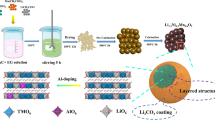
Sn-doped Li-rich layered oxides of Li1.2Mn0.54-x Ni0.13Co0.13Sn x O2 have been synthesized via a sol-gel method, and their microstructure and electrochemical performance have been studied. The addition of Sn4+ ions has no distinct influence on the crystal structure of the materials. After doped with an appropriate amount of Sn4+, the electrochemical performance of Li1.2Mn0.54-x Ni0.13Co0.13Sn x O2 cathode materials is significantly enhanced. The optimal electrochemical performance is obtained at x = 0.01. The Li1.2Mn0.53Ni0.13Co0.13Sn0.01O2 electrode delivers a high initial discharge capacity of 268.9 mAh g−1 with an initial coulombic efficiency of 76.5% and a reversible capacity of 199.8 mAh g−1 at 0.1 C with capacity retention of 75.2% after 100 cycles. In addition, the Li1.2Mn0.53Ni0.13Co0.13Sn0.01O2 electrode exhibits the superior rate capability with discharge capacities of 239.8, 198.6, 164.4, 133.4, and 88.8 mAh g−1 at 0.2, 0.5, 1, 2, and 5 C, respectively, which are much higher than those of Li1.2Mn0.54Ni0.13Co0.13O2 (196.2, 153.5, 117.5, 92.7, and 43.8 mAh g−1 at 0.2, 0.5, 1, 2, and 5 C, respectively). The substitution of Sn4+ for Mn4+ enlarges the Li+ diffusion channels due to its larger ionic radius compared to Mn4+ and enhances the structural stability of Li-rich oxides, leading to the improved electrochemical performance in the Sn-doped Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials.










Similar content being viewed by others
References
Goodennough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4:3243–3262
Wang Z, Luo S, Ren J, Wang D, Qi X (2016) Enhanced electrochemical performance of Li-rich cathode Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification with lithium ion conductor Li3PO4. Appl Surf Sci 370:437–444
Wang YX, Shang KH, He W, Ai XP, Cao YL, Yang HX (2015) Magnesium-doped Li1.2[Co0.13Ni0.13Mn0.54]O2 for lithium-ion battery cathode with enhanced cycling stability and rate capability. ACS Appl Mater Interfaces 7:13014–13021
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M= Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Ito A, Li D, Ohsawa Y, Sato Y (2008) A new approach to improve the high-voltage cyclic performance of Li-rich layered cathode material by electrochemical pre-treatment. J Power Sources 183:344–346
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15:2257–2267
Venkateswara Rao C, Soler J, Katiyar R, Shojan J, West WC, Katiyar RS (2014) Investigations on electrochemical behavior and structural stability of Li1.2Mn0.54Ni0.13Co0.13O2 lithium-ion cathodes viain-situ and ex-situ Raman spectroscopy. J Phys Chem C 118:14133–14141
Guo XJ, Li YX, Zheng M, Zheng JM, Li J, Gong ZL, Yang Y (2008) Structural and electrochemical characterization of xLi[Li1/3Mn2/3]O2•(1−x)Li[Ni1/3Mn1/3Co1/3]O2 (0≤x≤0.9) as cathode materials for lithium ion batteries. J Power Sources 184:414–419
Lu Z, Dahn JR (2002) Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem Soc 149:A815–A822
Johnson CS, Kim JS, Lefief C, Li N, Vaughey JT, Thackeray MM (2004) The significance of the Li2MnO3component in ‘composite’ xLi2MnO3•(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem Commun 6:1085–1091
Yu H, Wang Y, Asakura D, Hosono E, Zhang T, Zhou H (2012) Electrochemical kinetics of the 0.5Li2MnO3•0.5LiMn0.42Ni0.42Co0.16O2 ‘composite’ layered cathode material for lithium-ion batteries. RSC Adv 2:8797–8807
Zhang HZ, Qiao QQ, Li GR, Ye SH, Gao XP (2012) Surface nitridation of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as cathode material for lithium-ion battery. J Mater Chem 22:13104–13109
Armstrong AR, Holzapfel M, Novák P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J Am Chem Soc 128:8694–8698
Kang SH, Kempgens P, Greenbaum S, Kropf AJ, Amine K, Thackeray MM (2007) Interpreting the structural and electrochemical complexity of 0.5Li2MnO3•0.5LiMO2 electrodes for lithium batteries (M= Mn0.5−xNi0.5−xCo2x, 0≤x≤0.5). J Mater Chem 17:2069–2077
Johnson CS, Li N, Lefief C, Thackeray MM (2007) Anomalous capacity and cycling stability of xLi2MnO3·(1−x)LiMO2 electrodes (M = Mn, Ni, Co) in lithium batteries at 50 °C. Electrochem Commun 9(4):787–795
Manthiram A, Knight JC, Myung ST, Oh SM, Sun YK (2016) Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv Energy Mater 6:1501010
Liu X, Huang T, Yu A (2015) Surface phase transformation and CaF2 coating for enhanced electrochemical performance of Li-rich Mn-based cathodes. Electrochim Acta 163:82–92
Lu C, Wu H, Zhang Y, Liu H, Chen B, Wu N, Wang S (2014) Cerium fluoride coated layered oxide Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691
Kang SH, Johnson CS, Vaughey JT, Amine K, Thackeray MM (2006) The effects of acid treatment on the electrochemical properties of 0.5Li2MnO3∙0.5LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells. J Electrochem Soc 153:A1186–A1192
Kang SH, Thackeray MM (2008) Stabilization of xLi2MnO3·(1−x)LiMO2 electrode surfaces (M= Mn, Ni, Co) with mildly acidic fluorinated solutions. J Electrochem Soc 155:A269–A275
Wang D, Belharouak I, Ortega LH, Zhang X, Xu R, Zhou D, Zhou G, Amine K (2015) Synthesis of high capacity cathodes for lithium-ion batteries by morphology-tailored hydroxide co-precipitation. J Power Sources 274:451–457
Fu F, Deng YP, Shen CH, Xu GL, Peng XX, Wang Q, Xu YF, Fang JC, Huang L, Sun SG (2014) A hierarchical micro/nanostructured 0.5Li2MnO3·0.5LiMn0.4Ni0.3Co0.3O2 material synthesized by solvothermal route as high rate cathode of lithium ion battery. Electrochem Commun 44:54–58
He W, Yuan D, Qian J, Ai X, Yang H, Cao Y (2013) Enhanced high-rate capability and cycling stability of Na-stabilized layered Li1.2[Co0.13Ni0.13Mn0.54]O2 cathode material. J. Mater Chem A 1:11397–11403
Liu X, Liu J, Huang T, Yu A (2013) CaF2-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode materials for Li-ion batteries. Electrochim. Acta 109:52–58
Li Q, Li G, Fu C, Luo D, Fan J, Li L (2014) K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl Mater Interfaces 6:10330–10341
Wang D, Huang Y, Huo Z, Chen L (2013) Synthesize and electrochemical characterization of Mg-doped Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material. Electrochim. Acta 107:461–466
Zhao J, Wang Z, Guo H, Li X, He Z, Li T (2015) Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram Int 41(9):11396–11401
Zhang WH, He W, Pei F, Wu FY, Mao RJ, Ai XP, Yang HX, Cao YL (2013) Improved electrochemical properties of Al3+-doped 0.5Li2MnO3-0.5LiCo1/3Ni1/3Mn1/3O2 cathode for lithium ion batteries. J Inorg Mater 28:1261–1264
Jiao LF, Zhang M, Yuan HT, Zhao M, Guo J, Wang W, Zhou XD, Wang YM (2007) Effect of Cr doping on the structural, electrochemical properties of Li[Li0.2Ni0.2−x/2Mn0.6−x/2Cr x ]O2 (x = 0, 0.02, 0.04, 0.06, 0.08) as cathode materials for lithium secondary batteries. J Power Sources 167:178–184
Yan J, Liu X, Li B (2014) Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv 4:63268–63284
Song B, Lai MO, Lu L (2012) Influence of Ru substitution on Li-rich 0.55Li2MnO3·0.45LiNi1/3Co1/3Mn1/3O2 cathode for Li-ion batteries. Electrochim. Acta 80:187–195
Jin X, Xu Q, Liu H, Yuan X, Xia Y (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
He Z, Wang Z, Chen H, Huang Z, Li X, Guo H, Wang R (2015) Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J Power Sources 299:334–341
Xia Y, Shi S, Li C, Liang C, Gan Y, Huang H, Tao X, Zhang W (2015) Electrochemical properties of Sn-doped Li3V2(PO4)3 cathode material synthesized via a citric acid assisted sol-gel method. J Alloys Compd 652:298–306
Ma J, Li B, Du H, Xu C, Kang F (2011) Effects of tin doping on physicochemical and electrochemical performances of LiFe1−xSnxPO4/C (0 ≤ x ≤ 0.07) composite cathode materials. Electrochim. Acta 56:7385–7391
Ren H, Mu X, Huang Y, Li Z, Wang Y, Cai P, Peng Z, Zhou Y (2010) Effects of Sn doping on electrochemical characterizations of Li[Ni1/3Co1/3Mn1/3]O2 cathode material. Ionics 16:497–502
Zhao Y, Xia M, Hu X, Zhao Z, Wang Y, Lv Z (2015) Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim. Acta 174:1167–1174
Zhou L, Tian M, Deng Y, Zheng Q, Xu C, Lin D (2016) La2O3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with enhanced specific capacity and cycling stability for lithium-ion batteries. Ceram Int 42:15623–15633
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim. Acta 105:200–208
Sun L, Yi X, Ren X, Zhang P, Liu J (2016) Synthesis and electrochemical performances of Y-doped lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material. J Electrochem Soc 163:A766–A772
Han E, Li Y, Zhu L, Zhao L (2014) The effect of MgO coating on Li1.17Mn0.48Ni0.23Co0.12O2 cathode material for lithium ion batteries. Solid State Ionics 255:113–119
Yuan W, Zhang HZ, Liu Q, Li GR, Gao XP (2014) Surface modification of Li(Li0.17Ni0.2Co0.05Mn0.58)O2 with CeO2 as cathode material for Li-ion batteries. Electrochim Acta 135:199–207
Zhang Y, Hou P, Zhou E, Shi X, Wang X, Song D, Guo J, Zhang L (2015) Pre-heat treatment of carbonate precursor firstly in nitrogen and then oxygen atmospheres: a new procedure to improve tap density of high-performance cathode material Li1.167(Ni0.139Co0.139Mn0.556)O2 for lithium ion batteries. J Power Sources 292:58–65
Ma D, Li Y, Zhang P, Cooper AJ, Abdelkader AM, Ren X, Deng L (2016) Mesoporous Li1.2Mn0.54Ni0.13Co0.13O2 nanotubes for high-performance cathodes in Li-ion batteries. J Power Sources 311:35–41
Wang J, He X, Paillard E, Laszczynski N, Li J, Passerini S (2016) Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries. Adv. Energy Mater 6(21):1600906
Shi SJ, Tu JP, Mai YJ, Zhang YQ, Gu CD, Wang XL (2012) Effect of carbon coating on electrochemical performance of Li1.048Mn0.381Ni0.286Co0.286O2 cathode material for lithium-ion batteries. Electrochim Acta 63:112–117
Sun YY, Li F, Qiao QQ, Cao JS, Wang YL, Ye SH (2015) Surface modification of Li(Li0.17Ni0.2Co0.05Mn0.58)O2 with LiAlSiO4 fast ion conductor as cathode material for Li-ion batteries. Electrochim Acta 176:1464–1475
Qiao QQ, Qin L, Li GR, Wang YL, Gao XP (2015) Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as a cathode for advanced lithium-ion batteries. J Mater Chem A 3:17627–17634
Lee E, Koritala R, Miller DJ, Johnson CS (2015) Aluminum and gallium substitution into 0.5Li2MnO3·0.5Li(Ni0.375Mn0.375Co0.25)O2 layered composite and the voltage fade effect. J Electrochem Soc 162(3):A322–A329
Feng X, Gao Y, Ben L, Yang Z, Wang Z, Chen L (2016) Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries. J Power Sources 317:74–80
Zhang HZ, Li F, Pan GL, Li GR, Gao XP (2015) The effect of polyanion-doping on the structure and electrochemical performance of Li-rich layered oxides as cathode for lithium-ion batteries. J Electrochem Soc 162(9):A1899–A1904
Chen M, Chen D, Liao Y, Zhong X, Li W, Zhang Y (2016) Layered lithium-rich oxide nanoparticles doped with spinel phase: acidic sucrose-assistant synthesis and excellent performance as cathode of lithium ion battery. ACS Appl Mater Interfaces 8:4575–4584
Amalraj F, Talianker M, Markovsky B, Sharon D, Burlaka L, Shafir G, Zinigrad E, HaikO AD, Lampert J, Schulz-Dobrick M, Garsuch A (2013) Study of the lithium-rich integrated compound xLi2MnO3·(1-x)LiMO2 (x around 0.5; M= Mn, Ni, Co; 2: 2: 1) and its electrochemical activity as positive electrode in lithium cells. J Electrochem Soc 160(2):A324–A337
Ates MN, Mukerjee S, Abraham KM (2014) A Li-rich layered cathode material with enhanced structural stability and rate capability for Li-on batteries. J Electrochem Soc 161(3):A355–A363
Wang C, Zhou F, Chen K, Kong J, Jiang Y, Yan G, Li J, Yu C, Tang W (2015) Electrochemical properties of α-MoO3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. Electrochim Acta 176:1171–1181
He W, Qian J, Cao Y, Ai X, Yang H (2012) Improved electrochemical performances of nanocrystalline Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv 2(8):3423–3429
Li F, Sun YY, Yao ZH, Cao JS, Wang YL, Ye SH (2015) Enhanced initial coulombic efficiency of Li1.14Ni0.16Co0.08Mn0.57O2 cathode materials with superior performance for lithium-ion batteries. Electrochim Acta 182:723–732
Yang S, Huang G, Hu S, Hou X, Huang Y, Yue M, Lei G (2014) Improved electrochemical performance of the Li1.2Ni0.13Co0.13Mn0.54O2 wired by CNT networks for lithium-ion batteries. Mater Lett 118:8–11
Acknowledgements
This work was supported by the national college students’ innovation and entrepreneurship training program (201510636004), the outstanding graduate theses foundation of Sichuan Normal University (2016-4-32), and the large precision instrument projects of Sichuan Normal University (DJ2016-27).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(DOCX 471 kb)
Rights and permissions
About this article
Cite this article
Zhou, L., Liu, J., Huang, L. et al. Sn-doped Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials for lithium-ion batteries with enhanced electrochemical performance. J Solid State Electrochem 21, 3467–3477 (2017). https://doi.org/10.1007/s10008-017-3688-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3688-y