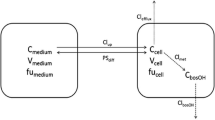
Abstract
Conjugation/deconjugation cycling plays an important role in the physiologic regulation of the concentration of endogenous compounds that form conjugated metabolites. Less is known concerning the deconjugation of xenobiotics. The model compound p-nitrophenol (pNP) is conjugated to sulfate and glucuronide metabolites which can also undergo hydrolysis, via separate enzyme systems, to regenerate pNP. In the present investigation, computer simulations were performed using literature values for theK M andV max for each of the four enzyme systems involved in net pNP conjugation. The apparent sulfation rate, apparent glucuronidation rate, and the extraction ratio (E) of pNP were each examined (i) as a function of pNP concentration, (ii) following alterations in theK M andV max values for the deconjugation enzymes, (iii) after modulating the enzyme distribution patterns along the liver flow path for both the conjugating and deconjugating enzymes, and (iv) in the presence of drug metabolite diffusional barriers for membrane transport. Results of these simulations demonstrated that changes in theK M orV max for deglucuronidation produced changes not only in net glucuronidation but also in net sulfation. Overall extraction (E) of the parent compound was only affected when glucuronidation was an important pathway, i.e., at higher pNP concentrations. Similar results were observed with changes in desulfation, with desulfation having the greatest effects at low pNP concentrations where sulfation represents the predominant metabolic pathway. Changes in the enzyme distribution patterns for the deconjugation pathways showed that the greatest influence on net conjugation rates occurred when hydrolase enzyme activity was distributed downstream from the respective forward reaction. In the presence of a diffusional barrier for metabolite transport (i.e., when the diffusional clearance was one tenth of blood flow), net metabolism of parent was diminished withE decreasing from 0.74, in the absence of a barrier, to 0.23, since the generated metabolite remained, to a great extent, within hepatocytes and underwent a more pronounced hydrolysis. In the presence of diffusional barriers for uptake of the conjugated metabolites, the lowest drug extraction and metabolite formation rates were observed when the distribution of the conjugation and deconjugation pathways across the liver were the same. Therefore, the effects of deconjugation on hepatic drug removal and metabolite formation are highly dependent on the enzymatic parameters of both the forward and reverse reactions, the parent drug concentration, the enzyme distribution patterns, and the presence of diffusional barriers for metabolite membrane transport. Since a change in the deconjugation of one metabolite can influence the net formation of not only itself but also other metabolites, and overall drug extraction, evaluation of conjugation/deconjugation cycling represents an important consideration in pharmacokinetic studies involving physiological-, pathological-, or pharmacological-induced alterations in conjugate formation.
Similar content being viewed by others
References
F. C. Kauffman. Regulation of drug conjugate production by futile cycling in intact cells. In F. C. Kauffman (ed.)Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, New York, 1994, pp. 243–255.
A. B. Roy. Sulfotransferases. In G. J. Mulder (ed.),Sulfation of Drugs and Related Compounds, CRC Press, Boca Raton, 1981, pp. 83–130.
A. B. Roy. Sulfatase, lysosomes, and disease.Aust. J. Exp. Biol. Med. Sci. 54: 111–135 (1976).
G. J. Dutton.Glucuronidation of Drugs and Other Compounds, CRC Press, Boca Raton, 1980, pp. 83–96.
A. A. Farooqui and P. Mandel. The clinical aspects of arylsulfatases.Clin. Chim. Acta 74:93–100 (1977).
R. Shankaran, M. Ameen, W. L. Daniel, R. G. Davidson, and P. L. Chang. Characterization of arylsulphatase C isozymes from human liver and placenta.Biochim. Biophys. Acta 1078:251–257 (1991).
K.-J. Ho, S.-C. Hsu, J.-S. Chen, and L.-H. C. Ho. Human biliary β-glucuronidase: Correlation of its activity with deconjugation of bilirubin in the bile.Eur. J. Clin. Invest. 16:361–367 (1986).
S. A. Belinsky, F. C. Kauffman, P. M. Sokolove, T. Tsukuda, and R. G. Thurman. Calcium-mediated inhibition of glucuronide production by epinephrine in the perfused rat liver.J. Biol. Chem. 259:7705–7711 (1984).
F. M. Brunelle and R. K. Verbeeck. Glucuronidation of diflunisol by rat liver microsomes. Effect of microsomal β-glucuronidase activity.Biochem. Pharmacol. 46:1953–1958 (1993).
I. M. Anundi, F. C. Kauffman, M. El-Mouelhi, and R. G. Thurman. Hydrolysis of organic sulfates in periportal and pericentral regions of the liver lobule: studies with 4-methylumbelliferyl sulfate in the perfused rat liver.Mol. Pharmacol. 29:599–605 (1986).
I. M. Anundi, F. C. Kauffman, M. El-Mouelhi, and R. G. Thurman. Hydrolysis of 4-methylumbelliferyl sulfate in periportal and pericentral areas of the liver lobule.Arch. Toxicol. 60:69–72 (1987).
S. Ratna, M. Chiba, L. Bandyopadhyay, and K. S. Pang. Futile cycling between 4-methylumbelliferone and its conjugates in perfused rat liver.Hepatology 17:838–853 (1993).
S. Miyauchi, Y. Sugiyami, T. Iga, and M. Hanano. The conjugative metabolism of 4-methylumbelliferone and deconjugation to the parent drug examined by the isolated perfused liver and in vitro liver homogenates of rats.Chem. Pharm. Bull. 37:475–480 (1989).
M. Whittaker, P. M. Sokolove, R. G. Thurman, and F. C. Kauffman. Stimulation of 3-benzo[α]pyrenyl glucuronide hydrolysis by calcium activation of microsomal β-glucuronidase.Cancer Lett. 26a:145–152 (1986).
P. M. Sokolove, M. A. Wilcox, R. G. Thurman, and F. C. Kauffman. Stimulation of hepatic microsomal β-glucuronidase by calcium.Biochem. Biophys. Res. Commun. 121:987–993 (1984).
I. Schöllhammer, D. S. Poll, and M. H. Bickel. Liver microsomal β-glucuronidase and UDP-glucuronyltransferase,Enzyme 20:269–276 (1975).
I. A. M. DeLannoy and K. S. Pang. Commentary: Presence of a diffusional barrier on metabolite kinetics: Enalaprilat as a generated versus preformed metabolite.Drug Metab. Dispos. 14:513–520 (1986).
S. Miyauchi, Y. Sugiyami, T. Iga, and M. Hanano. Membrane-limited hepatic transport of the conjugative metabolites of 4-methylumbelliferone in rats.J. Pharm. Sci. 77:688–692 (1988).
C. A. Goresky, K. S. Pang, A. J. Schwab, F. Barker III, W. F. Cherry, and G. G. Bach. Uptake of a protein bound polar compound, acetaminophen sulfate, by perfused rat liver.Hepatology 16:173–190 (1992).
D. W. Sundheimer and K. Brendel. Metabolism of harmol and transport of harmol conjugates in isolated rat hepatocytes.Drug Metab. Dispos. 11:433–440 (1983).
M. Vore. Regulation of drug conjugate processing by hepatocellular transport systems. In F. C. Kauffman (ed.),Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, New York, 1994, pp. 311–338.
I. A. M. DeLannoy and K. S. Pang. Effect of diffusional barriers on drug and metabolite kinetics.Drug Metab. Dispos. 15:51–58 (1987).
H. Sato, Y. Sugiyama, S. Miyauchi, Y. Sawada, T. Iga, and M. Hanano. A simulation study on the effect of a uniform diffusional barrier across hepatocytes on drug metabolism by evenly or unevenly distributed uni-enzyme in the liver.J. Pharm. Sci. 75:3–8 (1986).
S. Miyauchi, Y. Sugiyama, H. Sato, Y. Sawada, T. Iga, and M. Hanano. Effect of a diffusional barrier to a metabolite across hepatocytes on its kinetics in “enzyme-distributed” models: A computer-aided simulation study.J. Pharmacokin. Biopharm. 15:399–421 (1987).
F. C. Kauffman, M. Whittaker, I. Anundi, and R. G. Thurman. Futile cycling of a sulfate conjugate by isolated hepatocytes.Mol. Pharmacol. 39: 414–420 (1991).
A. Lombardo, G. Goi, E. Pistolesi, E. Rocca, S. Agosti, A. Fabi, A. Giuliani, A. B. Burlina, and G. Tettamanti. Behaviour of several enzymes of lysosomal orgin in human plasma during pregnancy,Clin. Chim. Acta 143: 253–264 (1984).
F. Belfiore, L. L. Vecchio, and E. Napoli. Serum beta-glucuronidase activity in diabetic patients as related to vascular complications and degree of glucose metabolic disorder.Am. J. Med. Sci. 264:457–466 (1972).
L. Kelly and S. R. Hall. Cathepsin D and aryl sulfatase activities in diabetic and insulin treated diabetic rat plasma, liver, kidney, and urine.Med. Sci. Res. 15:789–790 (1987).
M. E. Morris and K. S. Pang. Competition between two enzymes for substrate removal in liver: Modulating effects due to substrate recruitment of hepatocyte activity.J. Pharmacokin. Biopharm. 15:473–496 (1987).
M. E. Morris, V. Yuen, and K. S. Pang. Competing pathways in drug metabolism. II. An identical, anterior enzymatic distribution for 2- and 5-sulfoconjugation and a posterior localization for 5-glucuronidation of gentisamide in the rat liver.J. Pharmacokin. Biopharm. 16:633–656 (1988).
R. B. Hornbeck. The numerical solution of ordinary differential equations. InNumerical Methods, Quantum Publishers, New York, 1975, pp. 185–226.
P. M. Belanger, M. Lalande, G. Labrecque, and F. M. Dore. Diurnal variations in the transferases and hydrolases involved in glucuronide and sulfate conjugation of rat liver.Drug Metab. Dispos. 13:386–389 (1985).
P. I. Eacho, D. Sweeny, and M. Weiner. Conjugation ofp-nitroanisole andp-nitrophenol in hepatocytes from streptozotocin diabetic rats.J. Pharmacol. Exp. Ther. 218:34–40 (1981).
L. A. Reinke, S. A. Belinsky, R. K. Evans, F. C. Kauffman, and R. G. Thurman. Conjugation ofp-nitrophenol in the perfused rat liver: The effect of substrate concentration and carbohydrate reserves.J. Pharmacol. Exp. Ther. 217:863–870 (1981).
M. El Mouelhi and F. C. Kauffman. Sublobular distribution of transferases and hydrolases associated with glucuronide, sulfate, and glutathione conjugation in human liver.Hepatology 6:450–456 (1986).
K. S. Pang and M. Chiba. Pharmacokinetic modeling of drug conjugates. In F. C. Kauffman (ed.),Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, New York, 1994, pp. 257–309.
A. R. Pösö, K. E. Penttilä, and K. O. Lindros. Heterogeneous zonal distribution of lysosomal enzymes in rat liver.Enzyme 45:174–179 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hansel, S.B., Morris, M.E. Hepatic conjugation/deconjugation cycling pathways. Computer simulations examining the effect of michaelis-menten parameters, enzyme distribution patterns, and a diffusional barrier on metabolite disposition. Journal of Pharmacokinetics and Biopharmaceutics 24, 219–243 (1996). https://doi.org/10.1007/BF02353490
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02353490