Summary
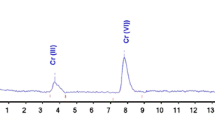
Chromium can be present in aqueous solution as Cr(VI) or in monomeric, dimeric, trimeric and higher polymeric forms of Cr(III). Many monomeric forms of Cr(III) are possible, with the water molecules of Cr(H2O) 3+6 substituted by anionic or neutral species. This proliferation of Cr(III) species makes the complete speciation of chromium a continuing challenge to the analyst. A simple and effective cation exchange procedure for the separation of various of these species uses a small glass column containing 1 mL of pre-treated cation exchange resin (Na+ form). Stepwise elution with solutions of perchloric acid, Ca2+ (pH=2) and La3+ (pH=2) separates Cr(VI) and seven Cr(III) species from CrX3 to tetramer. Radiometric (Cr-51), spectrophotometric and other detection methods can be employed; the use of radiochromium gives the lowest detection limit.
Similar content being viewed by others
References
“Chromium: the Medical and Biological Effects of Environmental Pollutants”, National Academy of Sciences, Washington, D.C., 1974.
R. E. Ackerhalt, C. H. Collins, K. E. Collins, Radiochim. Acta,14, 49 (1970).
R. E. Cranston, J. W. Murray, Anal. Chim. Acta,99, 275 (1978).
K. S. Subramanian, Anal. Chem.60, 11 (1988).
G. E. Gatley, J. P. Matousel, Anal. Chem.52, 1570 (1980).
S. Arpadjan, V. Krivan, Anal. Chem.58, 2611 (1986).
I. S. Krull, K. W. Panaro, L. L. Gershnan, J. Chromatogr. Sci.21, 460 (1983).
K. E. Lawrence, G. W. Price, V. A. Fassel, Anal. Chem.56 289 (1984).
G. Schwedt, Fresenius Z. Anal. Chem.295, 382 (1979).
T. Tande, J. E. Petersen, T. Torgrimsen, Chromatographia13, 607 (1980).
J. Rüter, U. P. Fislage, B. Neidhart, Chromatographia,19 62 (1984).
J. C. Macdonald, “Inorganic Chromatographic Analysis” John Wiley and Sons, New York, 1985, p. 340.
C. H. Collins, K. E. Collins, R. E. Ackerhalt, J. Radioanal Chem.8, 263 (1971).
K. E. Collins, C. Archundia, C. H. Collins, Quím. Nova,6 164 (1983).
H. Stünzi, W. Marty, Inorg. Chem.22, 2145 (1983).
A. C. Adams, J. M. Crook, F. Nockhoff, E. L. King, J. Am. Chem. Soc.90, 5761 (1968).
H. S. Gates, E. L. King, J. Am. Chem. Soc.80, 5011 (1958).
C. H. Collins, G. S. Mello, M. A. Basso, C. Archundia, K. E. Collins, Sci. Total Environ.70, 205 (1988).
P. Gütlich, G. Harbottle, Radiochim. Acta,5, 70 (1966).
M. Thompson, R. E. Connick, Inorg. Chem.20, 2279 (1981).
J. E. Finholt, “Chemistry of Some Hydrolyzed Cr(II) Polymers”, Report UCRL-8879, 1960.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collins, K.E., Bonato, P.S., Archundia, C. et al. Column chromatographic speciation of chromium for Cr(VI) and several species of Cr(III). Chromatographia 26, 160–162 (1988). https://doi.org/10.1007/BF02268143
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02268143