Abstract
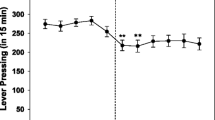
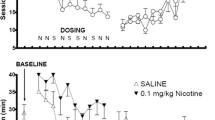
Rats responded under continuous reinforcement for 1%, 10% or 95% sucrose pellets, under food deprived or ad libitum access conditions. In both cases responding was highest for 10% sucrose reinforcement, and a small proportion of 95% sucrose was not consumed. Ad libitum food access reduced response rates for all sucrose concentrations. Responding for 10% and 95% sucrose pellets followed a parallel time-course; and accumulation of 95% sucrose pellets was immediate and nonprogressive. Extinction following availability of 95% sucrose pellets caused an increase in response rate, but removal of 10% sucrose led only to a decline in responding. Under both food deprived and non-deprived conditions, the dopamine D-2 antagonist raclopride dose-dependently decreased responding for 1% or 10% sucrose, but increased responding for, and consumption of, 95% sucrose reward. After eight sessions of responding under extinction conditions, the presentation of reward-associated cues increased response rate early in the session. Raclopride had no effect during this period, but decreased responding later in the session. We consider the implications of these results for theories of neuroleptic drug action.
Similar content being viewed by others
References
Bailey CS, Hsaio S, King JE (1986) Hedonic reactivity to sucrose in rats: Modification by pimozide. Physiol Behav 38:447–452
Berridge KC, Venier IL Robinson TE (1989) Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci 103:36–45
Colle LM, Wise RA (1988) Effects of nucleus accumbens amphetamine on lateral hypothalamic brain stimulation reward. Brain Res 459:361–368
de Paulis T, Kumar Y, Johansson L, Ramsby S, Hall H, Sallemark M, Angeby-Moller K, Ogren SO (1986) Potential neuroleptic agents. 4. Chemistry, behavioural pharmacology and blocking of3H-spiperone binding of 3,5-disubstituted N- [(1-ethyl-2-pyrrolidinyl)methyl] -6-methoxy-salicylamide. J Med Chem 29:61–69
Ferster CB, Skinner BF (1957) Schedules of reinforcement. Appleton-Century-Crofts, NY
Fouriezos G, Wise RA (1976) Pimozide-induced extinction of intracranial self-stimulation: response-patterns rule out motor performance deficits. Brain Res 103:377–380
Fowler SC (1990) Neuroleptics produce within-session response decrements: facts and theories. Drug Dev Res 20:101–116
Franklin KBJ, McCoy SN (1979) Pimozide-induced extinction in rats: Stimulus control of responding rules out a motor deficit. Pharmacol Biochem Behav 11:71–75
Gray T, Wise RA (1980) Effects of pimozide on lever-pressing behavior maintained on an intermittent reinforcement schedule. Pharmacol Biochem Behav 12:931–935
Guttman N (1953) Operant conditioning, extinction, and periodic reinforcement in relation to concentration of sucrose used as reinforcing agent. J Exp Psychol 46:213–224
Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42:1705–1712
Herrnstein RJ (1970) On the law of effect. J Exp Anal Behav 13:243–266
Heyman GM (1983) A parametric evaluation of the hedonic and motoric effects of drugs: pimozide and amphetamine. J Exp Anal Behav 40:113–122
Heyman GM, Kinzie DL, Seiden LS (1986) Chlorpromazine and pimozide alter reinforcement efficacy and motor performance. Psychopharmacology 88:346–353
Hillegaart V, Ahlenius S, Magnusson O, Fowler CJ (1987) Repeated testing of rats markedly enhances the duration of effects induced by haloperidol on treadmill locomotion, catalepsy, and a conditioned avoidance response. Pharmacol Biochem Behav 27:159–164
Hodos W, Valenstein ES (1962) An evaluation of response rate as a measure of rewarding intracranial stimulation. J Comp Physiol Psychol 53:502–508
Horvitz JC, Ettenberg A (1988) Haloperidol blocks the response-reinstating effects of food reward: a methodology for separating neuroleptic effects on reinforcement and motor processes. Pharmacol Biochem Behav 31:861–865
Hsaio S, Tuntland P (1971) Short-term satiety signals generated by saccharin and glucose solutions. Physiol Behav 7:287–289
Janssen PAJ, Niegemeers CJE, Schellekens KHL (1965) Is it possible to predict the clinical effects of neuroleptic drugs (major tranquilisers) from animal data? Arzneimittelforschung 15:104–117
Keller FS, Schoenfeld WN (1950) Principles of psychology. Appleton-Century-Crofts, NY
Kirkpatrick MA, Fowler SC (1989) Force-proportional reinforcement: Pimozide does not reduce rats' emission of higher forces for sweeter rewards. Pharmacol Biochem Behav 32:499–504
Kohler C, Hall H, Ogren SO, Gawell L (1985) Specific in vitro and in vivo binding of tritiated raclopride: a potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem Pharmacol 34:2251–2259
Martin-Iverson MT, Wilkie D, Fibiger HC (1987) Effects of haloperidol and d-amphetamine on perceived quantity of food and tones. Psychopharmacology 93:374–381
Mook DG, Bryner CA, Rainey LD, Wall CL (1980) Release of feeding by the sweet taste in rats: oropharyngeal satiety. Appetite 1:299–315
Mook DG, Kushner BD, Kushner LR (1981) Release of feeding by the sweet taste in rats: the specificity of oral satiety. Appetite 2:267–280
Morgan MJ (1974) Resistance to satiation. Anim Behav 22:449–466
Muscat R, Willner P (1989) Effects of selective dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacology 99:98–102
Phillips AG, Fibiger HC (1990) Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol 1:269–282
Phillips G, Willner P, Sampson D, Nunn J, Muscat R (1991a) Time-, schedule-, and reinforcer-dependent effects of pimozide and amphetamine. Psychopharmacology 104:125–131
Phillips G, Willner P, Muscat R (1991b) Reward-dependent suppression or facilitation of consummatory behaviour by raclopride. Psychopharmacology (in press)
Phillips G, Willner P, Muscat R (1991c) Anatomical substrates for neuroleptic-induced reward attenuation and neuroleptic-induced response decrement. Behav Pharmacol 2:129–141
Prado-Alcala R, Wise RA (1984) brain stimulation reward and dopamine terminal fields: I. Caudate-putamen, nucleus accumbens and amygdala. Brain Res 297:265–273
Rolls ET, Murzi E, Yaxley S, Thorpe S (1986) Sensory-specific satiety: Food-specific reduction in responsiveness of ventral forebrain neurons after feeding in the monkey. Brain Res 368:79–86
Rolls ET, Sienkiewicz ZJ, Yaxley S (1989) Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci 1:53–60
Sanger D (1986) Response decrement patterns after neuroleptic and non-neuroleptic drugs. Psychopharmacology 89:125–130
Seeman P (1980) Brain dopamine receptors. Pharmacol Rev 32:229–313
Taylor J, Robbins T (1986) 6-Hydroxydopamine lesions of the nucleus accumbens, but not the caudate nucleus, attenuate enhanced responding with reward-related stimuli by intra-accumbensd-amphetamine. Psychopharmacology 90:390–397
Thompson T, Heistad GT, Palermo DS (1963) Effect of amount of training on rate and duration of responding during extinction. J Exp Anal Behav 6:155–161
Tombaugh TN, Anisman H, Tombaugh J (1980) Extinction and dopamine receptor blockade after intermittent reinforcement training: failure to find functional equivalence. Psychopharmacology 70:19–28
Wilcove WG (1973) Ingestion affected by the oral environment: The role of gustatory adaptation on taste reactivity in the rat. Physiol Behav 11:297–312
Wiley JL, Porter JH, Faw WR (1989) Haloperidol blocks reacquisition of operant running during extinction following a single priming trial with food reward. Bull Psychon Soc 27:340–342
Willner P, Phillips G, Muscat R (1989) Time-dependent and schedule-dependent effects of DA receptor blockade. Behav Pharmacol 1:169–176
Willner P, Papp M, Phillips G, Maleeh M, Muscat R (1990) Pimozide does not impair sweetness discrimination. Psychopharmacology 102:278–282
Willner P, Phillips G, Muscat R (1991) Suppression of rewarded behaviour by neuroleptic drugs: can't or won't, or why? In: Willner P, Scheel-Kruger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, Chichester, pp 251–271
Wise RA, Spindler J, De Wit H, Gerber GJ (1978) Neuroleptic-induced ‘anhedonia’ in rats: pimozide blocks the reward quality of food. Science 201:262–264
Xenakis S, Sclafani A (1981) The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav 15:435–442
Young PT (1949) Studies of food preference, appetite, and dietary habit. IX. Palatability versus appetite as determinants of the critical concentrations of sucrose and sodium chloride. Comp Psychol Monogr 19:1–44
Young PT, Greene JT (1953) Quantity of food ingested as a measure of relative acceptibility. J Comp Physiol Psychol 46:288–294
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phillips, G., Willner, P. & Muscat, R. Suppression or facilitation of operant behaviour by raclopride dependent on concentration of sucrose reward. Psychopharmacology 105, 239–246 (1991). https://doi.org/10.1007/BF02244316
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244316