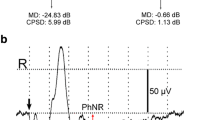
Abstract
Nine patients with maculopathy (macular holes, macular cysts, and lamellar holes) and ten patients with optic neuritis were examined in order to determine changes in the visual evoked potential (VEP) in response to pattern-reversal stimulation. Eyes with lamellar holes had normal P100 latency, but eyes with macular cysts and macular holes had prolonged P100 latency. Eyes with optic neuritis exhibited greater prolongation of the P100 latency than eyes with macular holes. In contrast, eyes with macular holes had a greater reduction in the steady-state VEP amplitude than eyes with optic neuritis. The prolonged latency occurring in maculopathy may be due to a peculiar amplitude summation noted with half-field VEP, rather than to a true conduction delay like that seen in eyes with optic neuritis. The amplitude slope, which is usually positive in normal controls, was negative for 85.7% of eyes with macular holes and 69.2% of eyes with optic neuritis. The negative amplitude slope may represent a subtle defect in retinal ganglion X cells. Eyes with significantly lower values for four or more of the nine central test points on quantitative automated perimetry had negative amplitude slopes and prolonged P100 latency.
Similar content being viewed by others
References
Bass SJ, Sherman J, Bodis-Wollner I, Nath S (1985) Visual evoked potentials in macular disease. Invest Ophthalmol Vis Sci 26:1071–1074
Blumhardt LD, Barrett G, Halliday AM, Kriss A (1978) The effect of experimental ‘scotomata’ on the ipsilateral and contralateral responses to pattern-reversal in one half-field. Electroencephalogr Clin Neurophysiol 45:376–392
Collins DWK, Carroll WM, Black JL, Walsh M (1979) Effect of refractive error on the visual evoked response. Br Med J 1:231–232
Douthwaite WA, Jenkins TCA (1982) Visually evoked responses to checkerboard patterns: check and field size interactions. Am J Optom Physiol Opt 59:894–901
Eves H (1975) Mensuration formulas. In: Selby SM (ed) Standard mathematical tables, 23rd edn. CRC Press, Ohio, p 2
Folk JC, Thompson HS, Han DP, Brown CK (1984) Visual function abnormalities in central serous retinopathy. Arch Ophthalmol 102:1299–1302
Fukui R, Kato M, Kuroiwa Y (1987) Effect of central scotomata on pattern reversal visual evoked potentials in patients with maculopathy and healthy subjects. Electroencephalogr Clin Neurophysiol (in press)
Harter MR, White CT (1970) Evoked cortical responses to checkerboard patterns: effect of check-size as a function of visual acuity. Electroencephalogr Clin Neurophysiol 28:48–54
Hauch TL, Straatsma BR, Hayreh MMS, Kreiger AE (1982) Macular holes: etiology and visual function. In: Henkind P (ed) ACTA: 24th International Congress of Ophthalmology. J.B. Lippincott, San Francisco, pp 477–480
Ikeda H (1980) Visual acuity, its development and amblyopia. J R Soc Med 73:546–555
Ikeda H, Wright MJ (1974) Is amblyopia due to inappropriate stimulation of the “sustained” pathway during development? Br J Ophthalmol 58:165–173
Ikeda H, Tremain KE, Sanders MD (1978) Neurophysiological investigation in optic nerve disease: combined assessment of the visual evoked response and electroretinogram. Br J Ophthalmol 62:227–239
Katz M (1984) The human eye as an optical system. In: Duane TD (ed) Clinical ophthalmology, vol 1. Harper and Row, Philadelphia, pp 9–16
Lennerstrand G (1982) Delayed visual evoked cortical potentials in retinal disease. Acta Ophthalmol 60:497–504
Marcus M, Merin S, Wolf M, Feinsod M (1983) Electrophysiological tests in assessment of senile macular degeneration. Ann Ophthalmol 15:235–238
McCormack GL, Tomlinson AA (1979) Human visual acuity assessment through linear extrapolation to threshold bar grating VERs. Am J Optom Physiol Opt 56:480–489
Meienberg O, Flammer J, Ludin HP (1982) Subclinical visual field defects in multiple sclerosis. Demonstration and quantification with automated perimetry, and comparison with visual evoked potentials. J Neurol 227:125–133
Nakamura Z (1979) Photopic and scotopic components of the human electroretinogram and visual evoked cortical potential. Jpn J Ophthalmol 23:289–300
Papakostopoulos D, Hart CD, Cooper R, Natsikos V (1984) Combined electrophysiological assessment of the visual system in central serous retinopathy. Electroencephalogr Clin Neurophysiol 59:77–80
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis. Guidelines for research protocols. Ann Neurol 13:227–231
Regan D (1978) Assessment of visual acuity by evoked potential recording: Ambiguity caused by temporal dependence of spatial frequency selectivity. Vision Res 18:439–443
Regan D, Richards W (1971) Independence of evoked potentials and apparent size. Vision Res 11:679–684
Sokol S (1978) Measurement of infant visual acuity from pattern reversal evoked potentials. Vision Res 18:33–39
Sokol S (1980) Pattern visual evoked potentials: Their use in pediatric ophthalmology. Int Ophthalmol Clin 20:251–268
Sokol S, Moskowitz A, Towle VL (1981) Age-related changes in the latency of the visual evoked potential: influence of check size. Electroencephalogr Clin Neurophysiol 51:559–562
Sokol S, Hansen VC, Moskowitz A, Greenfield P, Towle VL (1983) Evoked potential and preferential looking estimates of visual acuity in pediatric patients. Ophthalmology 90:552–562
Srebro R (1978) The visual evoked response. Binocular facilitation and failure when binocular vision is disturbed. Arch Ophthalmol 96:839–844
Stensaas SS, Eddington DK, Dobelle WH (1974) The topography and variability of the primary visual cortex in man. J Neurosurg 40:747–755
Straatsma BR, Foos RY, Spencer LM (1969) The retina — topography and clinical correlation. Symposium on retina and retinal surgery. Trans New Orleans Acad Ophthalmol. Mosby, St. Louis, pp 1–26
Wildberger H (1984) Neuropathies of the optic nerve and visual evoked potentials with special reference to color vision and differential light threshold measured with the computer perimeter Octopus. Doc Ophthalmol 58:147–227
Author information
Authors and Affiliations
Additional information
This study was supported in part by a Heed Ophthalmic Foundation Fellowship and a National Institutes of Health Giant EY 00331
Rights and permissions
About this article
Cite this article
Johnson, L.N., Yee, R.D., Hepler, R.S. et al. Alteration of the visual evoked potential by macular holes: Comparison with optic neuritis. Graefe's Arch Clin Exp Ophthalmol 225, 123–128 (1987). https://doi.org/10.1007/BF02160343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02160343