Abstract
Purpose
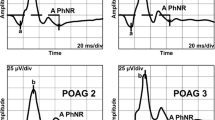
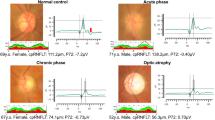
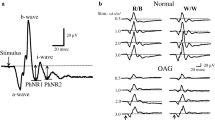
The photopic negative response (PhNR) is a negative wave following the b-wave of the photopic electroretinogram (ERG). The PhNR originates from the retinal ganglion cells (RGCs), and it can be used to assess the function of RGCs noninvasively and objectively. The purpose of this study was to determine whether the relative amplitudes (affected/normal eye) of the PhNR are significantly correlated with the degree of the relative afferent pupillary defect (RAPD) in eyes with unilateral or asymmetrical damage of the optic nerve.
Methods
The PhNRs of the full-field photopic ERGs were measured. In addition, videopupillography and automated perimetry were performed on 27 cases with asymmetrical optic nerve disorders including glaucoma. The differences of these assessments were expressed by the relative amplitudes of the PhNRs of the two eyes, the neutral density (ND) filter required to equate the amplitudes of the pupillary light reflexes between the two eyes, and differences of the mean defects (ΔMDs) of the sensitivities of the Humphrey visual fields. The correlations between these values were determined by linear regression analyses.
Results
The relative PhNR amplitudes were significantly and negatively correlated with the ΔMDs (R2 = 0.58, P = 0.0001). In addition, the relative PhNR amplitudes were moderately but significantly and positively correlated with the RAPDs (R2 = 0.36, P = 0.002).
Conclusion
The relative amplitudes of the PhNR of the affected eyes to the contralateral eyes indicate an asymmetric alteration of the RGCs, and they can be used to monitor the physiology of the RGCs objectively.


Similar content being viewed by others
Change history
15 June 2020
The original publication of this paper contains mistakes.
References
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Viswanathan S, Frishman LJ, Robson JG (2000) The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci 41(9):2797–2810
Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41(8):2205–2211
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346. https://doi.org/10.1001/archopht.122.3.341
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837. https://doi.org/10.1167/iovs.04-0458
Machida S, Gotoh Y, Tanaka M, Tazawa Y (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 137(5):938–940. https://doi.org/10.1016/j.ajo.2003.10.023
Bremner FD (2004) Pupil assessment in optic nerve disorders. Eye (Lond) 18(11)):1175–1181. https://doi.org/10.1038/sj.eye.6701560
Lagreze WD, Kardon RH (1998) Correlation of relative afferent pupillary defect and estimated retinal ganglion cell loss. Graefes Arch Clin Exp Ophthalmol 236(6):401–404. https://doi.org/10.1007/s004170050096
Kaback MB, Burde RM, Becker B (1976) Relative afferent pupillary defect in glaucoma. Am J Ophthalmol 81(4):462–468. https://doi.org/10.1016/0002-9394(76)90302-0
Lankaranian D, Altangerel U, Spaeth GL, Leavitt JA, Steinmann WC (2005) The usefulness of a new method of testing for a relative afferent pupillary defect in patients with ocular hypertension and glaucoma. Trans Am Ophthalmol Soc 103:200–207 discussion 20–208
Kalaboukhova L, Fridhammar V, Lindblom B (2007) Relative afferent pupillary defect in glaucoma: a pupillometric study. Acta Ophthalmol Scand 85(5):519–525. https://doi.org/10.1111/j.1600-0420.2006.00863.x
Tatham AJ, Meira-Freitas D, Weinreb RN, Marvasti AH, Zangwill LM, Medeiros FA (2013) Estimation of retinal ganglion cell loss in glaucomatous eyes with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci 55(1):513–522. https://doi.org/10.1167/iovs.13-12921
Waisbourd M, Lee B, Ali MH, Lu L, Martinez P, Faria B, Williams A, Moster MR, Katz LJ, Spaeth GL (2015) Detection of asymmetric glaucomatous damage using automated pupillography, the swinging flashlight method and the magnified-assisted swinging flashlight method. Eye (Lond) 29(10):1321–1328. https://doi.org/10.1038/eye.2015.106
Rukmini AV, Milea D, Baskaran M, How AC, Perera SA, Aung T, Gooley JJ (2015) Pupillary responses to high-irradiance blue light correlate with glaucoma severity. Ophthalmology 122(9):1777–1785. https://doi.org/10.1016/j.ophtha.2015.06.002
Wakakura M, Yokoe J (1995) Evidence for preserved direct pupillary light response in Leber’s hereditary optic neuropathy. Br J Ophthalmol 79(5):442–446. https://doi.org/10.1136/bjo.79.5.442
Utsumi T (1978) Open-loop infrared videopupillography—a preliminary report. Acta Soc Ophthalm Jpn 82(4):315–321
Ozeki N, Yuki K, Shiba D, Tsubota K (2013) Pupillographic evaluation of relative afferent pupillary defect in glaucoma patients. Br J Ophthalmol 97(12):1538–1542. https://doi.org/10.1136/bjophthalmol-2013-303825
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207. https://doi.org/10.1167/iovs.07-0887
Shen X, Huang L, Fan N, He J (2013) Relationship among photopic negative response, retinal nerve Fiber layer thickness, and visual field between normal and POAG eyes. ISRN Ophthalmol 2013:182021. https://doi.org/10.1155/2013/182021
Quigley HA, Addicks EM, Green WR (1982) Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol 100(1):135–146. https://doi.org/10.1001/archopht.1982.01030030137016
Kerrison JB, Buchanan K, Rosenberg ML, Clark R, Andreason K, Alfaro DV, Grossniklaus HE, Kerrigan-Baumrind LA, Kerrigan DF, Miller NR, Quigley HA (2001) Quantification of optic nerve axon loss associated with a relative afferent pupillary defect in the monkey. Arch Ophthalmol 119(9):1333–1341. https://doi.org/10.1001/archopht.119.9.1333
Johnson LN, Hill RA, Bartholomew MJ (1988) Correlation of afferent pupillary defect with visual field loss on automated perimetry. Ophthalmology 95(12):1649–1655. https://doi.org/10.1016/s0161-6420(88)32962-3
Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ (1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14(7):4368–4374
Li B, Barnes GE, Holt WF (2005) The decline of the photopic negative response (PhNR) in the rat after optic nerve transection. Doc Ophthalmol 111(1):23–31. https://doi.org/10.1007/s10633-005-2629-8
Karanjia R, Berezovsky A, Sacai PY, Cavascan NN, Liu HY, Nazarali S, Moraes-Filho MN, Anderson K, Tran JS, Watanabe SE, Moraes MN, Sadun F, DeNegri AM, Barboni P, do Val Ferreira Ramos C, La Morgia C, Carelli V, Belfort R Jr, Coupland SG, Salomao SR, Sadun AA (2017) The photopic negative response: an objective measure of retinal ganglion cell function in patients with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 58(6):BIO300–BIO306. https://doi.org/10.1167/iovs.17-21773
Tang J, Edwards T, Crowston JG, Sarossy M (2014) The test-retest reliability of the photopic negative response (PhNR). Transl Vis Sci Technol 3(6):1. https://doi.org/10.1167/tvst.3.6.1
Acknowledgments
The authors thank Duco Hamasaki, PhD, Bascom Palmer Eye Institute, University of Miami School of Medicine, for discussions and for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of Osaka Medical College and the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with animals performed by any of the authors.
Statement of informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okuno, T., Kida, T., Ikeda, T. et al. Significant correlations between photopic negative response, afferent pupillary defect, and mean defects of visual fields in asymmetric optic nerve disorders. Graefes Arch Clin Exp Ophthalmol 258, 1821–1827 (2020). https://doi.org/10.1007/s00417-020-04632-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04632-9