Abstract
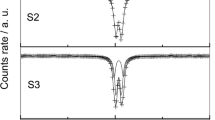
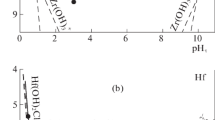
Sodium carbonate Na2CO3 is added to a solution containing an Fe(OH)2 precipitate in order to study the influence of CO 2−3 ions on the oxidation of ferrous hydroxide. The first stage of the reaction leads to a ferrous-ferric compound, the carbonate green rust one (GR1), identified by its X-ray diffraction pattern. The Mössbauer spectrum at 78 K of this GR1 displays two ferrous doublets and one ferric doublet in the 3∶1∶2 abundance ratio. The quadrupole splittingsQS are 2.91, 2.58 and 0.42 mm/s, respectively, and the isomer shifts 75 are 1.25, 1.25 and 0.47 mm/s respectively. These values are very close to those of the three doublets of the chloride GR1, 3Fe(OH)2Cl·Fe(OH)2Cl·nH2O. This fact confirms that the crystallographic structures of these two GR1s are similar, formed by the stacking of hydroxide layers and interlayers containing the considered anions (Cl− or CO 2−3 ) and water molecules. The chemical formula of carbonate GR1 is Fe (II)4 Fe (III)2 (OH)12CO3·nH2O, and its standard chemical potential -853 900 cal/mol ifn=0. The second stage of the reaction is the oxidation of GR1, which leads to α-FeOOH goethite.
Similar content being viewed by others
References
Ph. Refait and J.-M.R. Génin, Corros. Sci. 34(1993)797.
R.M. Taylor, Clay Minerals 15(1980)369.
J.-M.R. Génin, D. Rézel, Ph. Bauer, A. Olowe and A. Béral, Mat. Sci. Forum 8(1986)477.
A.A. Olowe and J.-M.R. Génin, Corros. Sci. 32(1991)965.
E. Murad and R.M. Taylor, Clay Minerals 19(1984)77.
E. Murad and R.M. Taylor, Hyp. Int. (1986)585.
R.C. West (ed.),Handbook of Chemistry and Physics, 69th Ed. (1988–1989).
R.M. Garrels and C.L. Christ,Equilibres des Minéraux et de leurs Solutions Aqueuses (Gauthier-Villars, Paris, 1967).
P.P. Stampfl, Corros. Sci. 9(1969)185.
R. Allmann, Chimia 24(1970)99.
R. Allmann, Acta Cryst. B24(1968)972.
Ph. Refait, D. Rézel, A.A. Olowe and J.-M.R. Génin, Hyp. Int. 69(1991)839.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drissi, H., Refait, P. & Génin, J.M.R. The oxidation of Fe(OH)2 in the presence of carbonate ions: Structure of carbonate green rust one. Hyperfine Interact 90, 395–400 (1994). https://doi.org/10.1007/BF02069145
Issue Date:
DOI: https://doi.org/10.1007/BF02069145