Abstract
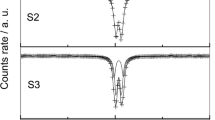
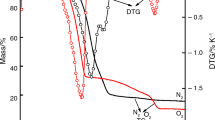
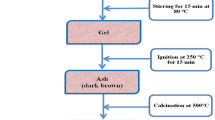
The phase analysis and nanostructural properties of the precipitates formed by forced hydrolysis of FeCl3 solutions at 160 °C in the presence of Cr3+ ions and hexamethylenetetramine (HMTA) were investigated. The phase analyses of the precipitates showed the presence of ferrihydrite (amorphous-like), α-FeOOH, β-FeOOH, and α-Fe2O3. The exact phase composition was strongly dependent on the starting chemical composition in the solution and the precipitation time. Ferrihydrite, α-FeOOH, and β-FeOOH were the precursors of α-Fe2O3 as the end-product. Upon 2 h of autoclaving at 160 °C, the precipitates consisted of nanoparticles ~ 35 to ~ 50 nm in size, whereas after 24 h their size increased to ~ 100 nm. New shapes of these nanoparticles were found. In these precipitation systems crystalline chromium (hydrous)oxide phase was not detected. However, the formation of solid solutions between Cr3+ ions and iron oxides could not be excluded taking into account Energy-dispersive X-ray spectroscopy (EDS) measurements.
Graphical abstract







Similar content being viewed by others
Data availability
Data presented in this study are available on requirement from corresponding author.
References
R.M. Cornell, U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions and Uses, 2nd edn. (Wiley, Weinheim, 2003)
S. Musić, M. Ristić, S. Krehula, 57Fe Mössbauer spectroscopy in the investigation of the precipitation of iron oxides, in Mössbauer Spectroscopy: Applications in Chemistry, Biology and Nanotechnology. ed. by V.K. Sharma, G. Klingelhöfer, T. Nishida (Wiley, Hoboken, 2013), pp.470–504. https://doi.org/10.1002/9781118714614
S. Musić, A. Šarić, S. Popović, Effects of urotropin on the formation of β-FeOOH. J. Mol. Struct. 410–411, 153–156 (1997). https://doi.org/10.1016/S0022-2860(96)09576-2
A. Šarić, K. Nomura, S. Popović, N. Ljubešić, S. Musić, Effects of urotropin on the chemical and microstructural properties of Fe-oxide powders prepared by the hydrolysis of aqueous FeCl3 solutions. Mater. Chem. Phys. 52, 214–220 (1998). https://doi.org/10.1016/S0254-0584(97)02032-4
A. Šarić, S. Musić, K. Nomura, S. Popović, FT-IR and 57Fe Mössbauer spectroscopic investigation of oxide phases precipitated from Fe(NO3)3 solutions. J. Mol. Struct. 480–481, 633–636 (1999). https://doi.org/10.1016/S0022-2860(98)00829-1
E.A. Dolgopolova, O.S. Ivanova, F.Y. Sharikov, V.K. Ivanov, A.E. Baranchikov, A.B. Shcherbakov, Y.D. Trietyakov, Microwave-hydrothermal synthesis of gadolinium-doped nanocrystalline ceria in the presence of hexamethylenetetramine. Russ. J. Inorg. Chem. 57, 1303–1307 (2012). https://doi.org/10.1134/S003602361210004X
H. Wang, C. Xie, D. Zeng, Z. Yang, Controlled organization of ZnO building blocks into complex nanostructures. J. Colloid Interface Sci. 297, 570–577 (2006). https://doi.org/10.1016/j.jcis.2005.10.059
S. Anas, R.V. Mangalaraja, S. Ananthakumar, Studies on the evolution of ZnO morphologies in a thermohydrolysis technique and evaluation of their functional properties. J. Hazard. Mater. 175, 889–895 (2010). https://doi.org/10.1016/j.jhazmat.2009.10.093
G. Ma, S. Salahub, C. Montemagno, S. Abraham, Highly active magnesium oxide nano materials for the removal of arsenates and phosphates from aqueous solutions. Nano-Struct. Nano-Objects 13, 74–81 (2018). https://doi.org/10.1016/j.nanoso.2017.11.006
J.L. Collins, M.H. Lloyd, R.L. Fellows, The basic chemistry involved in the internal-gelation method of precipitating uranium as determined by pH measurements. Radiochim. Acta 42, 121–134 (1987). https://doi.org/10.1524/ract.1987.42.3.121
M. Robić, M. Ristić, S. Krehula, M. Jurić, S. Musić, Synthesis of nanocrystalline eskolaite via grimaldiite. Chem. Pap. 75, 735–741 (2021). https://doi.org/10.1007/s11696-020-01338-4
S. Musić, Sorption of chromium (VI) and chromium (III) on aluminium hydroxide. J. Radioanal. Nucl. Chem. 100, 185–196 (1986)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. A 32, 751–767 (1976). https://doi.org/10.1107/S0567739476001551
M. Ristić, E. De Grave, S. Musić, S. Popović, Z. Orehovec, Transformation of low crystalline ferrihydrite to α-Fe2O3 in the solid state. J. Mol. Struct. 834–836, 454–460 (2007). https://doi.org/10.1016/j.molstruc.2006.10.016
S. Krehula, S. Musić, Influence of aging in an alkaline medium on the microstructural properties of α-FeOOH. J. Cryst. Growth 310, 513–520 (2008). https://doi.org/10.1016/j.jcrysgro.2007.10.072
C.J. Serna, M. Ocaña, J.E. Iglesias, Optical properties of α-Fe2O3 microcrystals in the infrared. J. Phys. C Solid State Phys. 20, 473–484 (1987). https://doi.org/10.1088/0022-3719/20/3/017
Y. Wang, A. Muramatsu, T. Sugimoto, FTIR analysis of well-defined α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 134, 281–297 (1998). https://doi.org/10.1016/S0927-7757(97)00102-7
M. Žic, M. Ristić, S. Musić, The effect of temperature on the crystallization of α-Fe2O3 particles from dense β-FeOOH suspensions. Mater. Chem. Phys. 120, 160–166 (2010). https://doi.org/10.1016/j.matchemphys.2009.10.040
B. Weckler, H.D. Lutz, Lattice vibration spectra. Part XCV. Infrared spectroscopic studies on the iron oxide hydroxides goethite (α), akaganéite (β), lepidocrocite (γ), and feroxyhite (δ). Eur. J. Solid State Inorg. Chem. 35, 531–544 (1998). https://doi.org/10.1016/S0992-4361(99)80017-4
E. Murad, J. Cashion, Mössbauer Spectroscopy of Environmental Materials and their Industrial Utilization (Kluwer Academic Publishers, Boston, Dordrecht, New York, London, 2004)
M. Robić, M. Ristić, E. Kuzmann, Z. Homonnay, S. Krehula, S. Musić, Forced hydrolysis of FeCl3 solutions in the presence of Cr3+ ions. J. Phys. Chem. Solids 156, 110166 (2021). https://doi.org/10.1016/j.jpcs.2021.110166
Acknowledgments
This work was supported by the Croatian Science Foundation (Grant No. IP-2016-06-8254).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented at the ACS Fall National Meeting, Atlanta, Georgia, USA, August 22–26 2021.
Zoltán Homonnay was a guest editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robić, M., Ristić, M., Krehula, S. et al. Forced hydrolysis of FeCl3 solutions in the presence of Cr3+ ions and hexamethylenetetramine. Journal of Materials Research 38, 1048–1060 (2023). https://doi.org/10.1557/s43578-022-00756-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00756-2