Summary
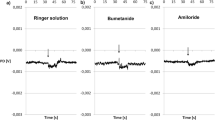
The potential dependence of unidirectional36Cl fluxes through toad skin revealed activation of a conductive pathway in the physiological region of transepithelial potentials. Activation of the conductance was dependent on the presence of Cl− or Br− in the external bathing solution, but was independent of whether the external bath was NaCl-Ringer's, NaCl-Ringer's with amiloride, KCl-Ringer's or choline Cl-Ringer's To partition the routes of the conductive Cl− ion flow, we measured in the isolated epithelium with double-barrelled microelectrodes apical membrane potentialV a , and intracellular Cl− activity,a cCl , of the principal cells indentified by differential interference contrast microscopy. Under short-circuit conditionsI sc=27.0±2.0 μA/cm2, with NaCl-Ringer's bathing both surfaces,V a was −67.9±3.8mV (mean ±se,n=24, six preparations) anda cCl was 18.0±0.9mM in skins from animals adapted to distilled water. BothV a anda aCl were found to be positively correlated withI sc (r=0.66 andr=0.70, respectively). In eight epithelia from animals adapted to dry milieu/tap waterV a anda cCl were measured with KCl Ringer's on the outside during activation and deactivation of the transepithelial Cl− conductance (G Cl) by voltage clamping the transepithelial potential (V) at 40 mV (mucosa positive) and −100 mV. AtV=40 mV; i.e. whenG Cl was deactivated,V a was −70.1±5.0 mV (n=15, eight preparations) anda cCl was 40.0±3.8mm. The fractional apical membrane resistance (fR a) was 0.69±0.03. Clamping toV=−100 mV led to an instantaneous change ofV a to 31.3±5.6 mV (cell interior positive with respect to the mucosal bath), whereas neithera cCl norfR a changed significantly within a 2 to 5-min period during whichG Cl increased by 1.19±0.10 mS/cm2. WhenV was stepped back to 40 mV,V a instantaneously shifted to −67.8±3.9 mV whilea cCl andfR a remained constant during deactivation ofG Cl. Similar results were obtained in epithelia impaled from the serosal side. In 12 skins from animals adapted to either tap water or distilled water the density of mitochondria-rich (D MRC) cells was estimated and correlated with the Cl current (I Cl though the fully activated (V=−100mV) Cl− conductance). A highly significant correlation was revealed (r=−0.96) with a slope of −2.6 nA/m.r. (mitochondria-rich cell and an I-axis intercept not significantly different from zero. In summary, the voltage-dependent Cl currents were not reflected infR a anda aCl of the principal cells but showed a correlation with the m.r. cell density. We conclude that the pricipal cells do not contribute significantly to the voltage-dependent Cl conductance.
Similar content being viewed by others
References
Armstrong, W. McD., Garcia-Diaz, J.F. 1981. Criteria for the use of microelectrodes to measure membrane potentials in epithelial cells.In: Epithelial Ion and Water Transport. A.D.C. Macknight and L.P. Leader, editors. pp. 43–53 Raven, New York
Biber, T.U.L., Drewnowska, K., Baumgarten, C.M., Fisher, R.S. 1985. Intracellular Cl activity changes of frog skin.Am. J. Physiol. 249:F432-F438
Brown, D., Grosso, A., De Sousa, R.C. 1981. The amphibian epidermis: Distribution of mitochondria-rich cells and the effect of oxytocin.J. Cell. Sci. 52:197–213
Bruus, K., Kristensen, P., Larsen, E.H. 1976. Pathways for chloride and sodium transport across toad skin.Acta Physiol. Scand. 97:31–47
Candia, O.A. 1978. Reduction of chloride fluxes by amiloride across the short-circuited frog skin.Am. J. Physiol. 234:F437-F445
Carasso, N., Favard, P., Jard, S., Rajerison, R.M. 1971. The isolated frog skin epithelium. I. Preparation and general structure in different physiological states.J. Microscopie 10:315–330
Curran, P.F. 1972. Effect of silver ion on permeability of frog skin.Biochim. Biophys. Acta 288:90–97
Davis, C.W., Finn, A.L. 1982. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia.Science 216:525–527
Dörge, A., Rick, R., Beck, F., Thurau, K. 1985. Cl transport across the basolateral membrane in frog skin epithelium.Pfluegers Arch. 405:S8-S11
Drewnowska, K., Biber, T.U.L. 1985. Active transport and exchange diffusion of Cl across the isolated skin ofRana pipiens.Am. J. Physiol. 249:F424–431
Dürr, J.E., Larsen, E.H. 1986. Indacrinone (MK-196)—A specific inhibitor of the voltage dependent Cl− permeability in toad skin.Acta Physiol. Scand. 127:145–153
Ehrenfeld, J., Masoni, A., Garcia-Romeu F. 1976. Mitochondria-rich cells of frog skin in transport mechanisms: Morphological and kinetic studies on transepithelial excretion of methylene blue.Am. J. Physiol. 231:120–126
Ferreira, K.T.G., Ferreira, H.G. 1981. The regulation of volume and ion composition in frog skin.Biochim. Biophys. Acta 646:193–202
Forkett, K.J., Bern, H.A., Machen, T.E., Conner, M. 1983. Chloride cells and the hormonal control of teleost fish osmoregulation.J. Exp. Biol. 106:255–281
Frizzell, R.A., Field, M., Schultz, S.G. 1979. Sodium-coupled chloride transport by epithelial tissues.Am. J. Physiol. 236:F1-F8
Fuchs, W., Larsen, E.H., Lindemann, B. 1977. Current-voltage curve of sodium channels and concentrations dependence of sodium permeability in frog skin.J. Physiol. (London) 267:137–166
Garcia-Diaz, J.F., Baxendale, L.M., Klemperer, G., Essig, A. 1985. Cell K activitt in frog skin in the prsence and absence of cell current.J. Membrane Biol. 85:143–158
Geck, P., Pietrzyk, C., Burckhardt, B.C., Pfeiffer, B., Heinz, E. 1980. Electrically silent cotransport of Na+, K+, Cl− in Ehrlich cells.Biochim. Biophys. Acta 600:432–447
Giraldez, F., Ferreira, K.T.G. 1984. Intracellular chloride activity and membrane potential in stripped frog skin (Rana temporaria).Biochim. Biophys. Acta 769:625–628
Harck, A., Larsen, E.H. 1986. Concentration dependence of halide fluxes and selectivity of the anion pathway in toad skin.Acta Physiol. Scand. 128:289–304
Harvey, B.J., Kernan, R.P. 1984. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain.J. Physiol. (London) 349:501–517
Helman, S.I., Fisher, R.S. 1977. Microelectrode studies of the active Na transport pathway of frog skin.J. Gen. Physiol. 69:571–604
Hille, B. 1984. Ionic channels of excitable membranes. pp. 1–426 Simauer Associates. Sunderland, Massachusetts
Hoffman, E.K., Sjøholm, C., Simonsen, L.O. 1983. Na+, Cl− cotransport in Ehrlich ascites tumor cells activated during volume regulation (regulatory volume increase).J. Membran Biol. 76:269–280
Katz, U., Larsen, E.H. 1984. Chloride transport in toad skin (Bufo viridis). The effect of salt adaptation.J. Exp. Biol. 109:353–372
Klyce, S.D., Marshall, W.S. 1982. Effects of Ag+ on ion transport by the corneal epithelium of the rabbit.J. Membrane Biol. 66:133–144
Koefoed-Johnsen, V., Levi, H., Ussing, H.H. 1952a. The mode of passage of chloride ions through the isolated frog skin.Acta Physiol. Scand. 25:150–163
Koefoed-Johnsen, V., Ussing, H.H., Zerahn, K. 1952b. The origin of the short-circuit current in the adrenaline stimulated frog skin.Acta Physiol. Scand. 27:38–48
Kristensen, P. 1978. Effect of amiloride on chloride tranport across amphibian epithelia.J. Membrane Biol. Special Issue:167–185
Kristensen, P. 1981. Is chloride transfer in frog skin localized to a special cell type.Acta Physiol. Scand. 113:123–124
Kristensen, P. 1982. Chloride transport in frog skin.In: Chloride Transport in Biological Membranes. J.A. Zadunaisky, editor. pp. 319–332 Academic, New York
Kristensen, P. 1983. Exchange diffusion, electrodiffusion, and rectification in the chloride transport pathway of frog skin.J. Membrane Biol. 72:141–151
Larsen, E.H., Kristensen, P. 1978. Properties of a conductive cellular chloride pathway in the skin of the toad (Bufo bufo).Acta Physiol. Scand. 102:1–21
Larsen, E.H., Rasmussen, B.E. 1982. Chloride channels in toad skin.Philos. Trans. R. Soc. London B 299:413–434
Li, J.H.-Y., Palmer, L.G., Edelman, I.S., Lindemann, B. 1982. The role of sodium-channel density in the natriferic response of the toad urinary bladder to an antidiuretic hormone.J. Membrane Biol. 64:77–89
Macey, R.I., Meyers, S. 1963. Dependence of chloride permeability on sodium in the isolated frog skin.Am. J. Physiol. 204:1095–1099
MacRobbie, E.A.C., Ussing, H.H. 1961. Osmotic behaviour of the epithelial cells of frog skin.Acta Physiol. Scand. 53:348–365
McCaig, D., Leader, J.P. 1984. Intracellular chloride activity in Extensor Digitorum Longus (EDL) muscle of the rat.J. Membrane Biol. 81:9–17
Nagel, W. 1975. Intracellular PD of frog skin epithelium.Pfluegers Arch. 355:R70
Nagel, W. 1976. The intracellular electrical potential profile of the frog skin epithelium.Pfluegers Arch. 365:135–143
Nagel, W. 1977. The dependence of the electrical potentials across the membranes of the frog skin upon the concentration of sodium in the mucosal medium.J. Physiol. (London) 269:777–796
Nagel, W. 1978. Effects of antidiuretic hormone upon electrical potential and resistance of apical and basolateral membranes of frog skin.J. Membrane Biol. 42:99–122
Nagel, W. 1979. Microelectrode artifacts and frog skin potentials. (Letters to the editor).J. Membrane Biol. 51:97–100
Nagel, W., Garcia-Diaz, J.F., Armstrong, W.McD. 1981. Intracellular ionic activities in frog skin.J. Membrane Biol.61:127–134
Nagel, W., Hirschmann, W. 1980. K+-permeability of the outer border of the frog skin (R. temporaria).J. Membrane Biol. 52:107–113
Nelson, D.J., Ehrenfeld, J., Lindemann, B. 1978. Volume changes and potential artifacts of epithelial cells of frog skin following impalement with microelectrodes filled with 3m KCl.J. Membrane Biol. Special Issue:91–99
Philpott, C.W. 1965. Halide localization in the teleost chloride cell and it's identification by selected area electron diffraction. Direct evidence supporting an osmoregulatory fuction for the seawater adapted chloride cel ofFundulus.Protoplasma 60:7–23
Ques-von Petery, M.V., Rotunno, C.A., Cereijido, M. 1978. Studies on chloride permeability in the skin ofLeptodactylus ocellatus: I. Na+ and Cl− effect on passive movements of Cl−.J. Membrane Biol. 42:317–330
Rick, R., Dörge, A., Arnim, E. von, Thurau, K. 1978. Electron microprobe analysis of frog skin epithelium: Evidence for a syncytial sodium transoort compartment.J. Membrane Biol. 39:313–331
Rick, R., Dörge, A., Katz, U., Bauer, R., Thurau, K. 1980. The osmotic behaviour of toad skin epithelium (Bufo viridis).Pfluegers Arch. 385:1–10
Rudneff, M. 1865. Uber die epidermoidale Schicht der Froschhau.Arch. Mikrosk. Anat. 1:295–298
Scheffey, C., Foskett, J.K., Machen, T.E. 1983. Localization of ionic pathways in the teleost opercular membrane by extracellular recording with a vibrating probe.J. Membrane Biol. 75:193–203
Spring, K.R., Kimura, G. 1978. Chloride reabsorption by renal proximal tubules ofNecturus.J. Membrane Biol. 38:233–254
Stoddard, J.S., Helman, S.I. 1982. Chloride efflux from isolated epithelia of frog skin.Fed. Proc. 41:1496
Stoddard, J.S., Jakobsson, E., Helman, S.I. 1985. Basolateral membrane chloride transport in isolated epithelia of frog skin.Am. J. Physiol. 249:C318-C329
Thompson, I.G., Mills, J.W. 1983. Chloride transport in glands of frog skin.Am. J. Physiol. 244:C221-C226
Ussing, H.H. 1949. The distinction by means of tracers between active transport and diffusion.Acta Physiol. Scand. 19:43–56
Ussing, H.H. 1982. Volume regulation of frog skin epithelium.Acta Physiol. Scand. 114:363–369
Ussing, H.H. 1985. Volume regulation and basolateral co-transport of sodium, potassium, and chloride ions in frog skin epithelium.Pfluegers Arch. 405:S2-S7
Ussing, H.H., Zerahn, K. 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin.Acta Physiol. Scand. 23:109–127
Van Driessche, W., Zeiske, W. 1980. Ba2+-induced conductance flactuations of spontaneously fluctuating K+ channels in the apical membrane of frog skin (Rana temporaria).J. Membrane Biol. 56:31–42
Voûte, C.L., Meier, W. 1978. The mitochondira-rich cell of frog skin as hormone-sensitive „shunt path” J. Membrane Biol.Speical Issue:151–165
Whitear, M. 1975. Flask cells and epidermal dynamics in frog skin.J. Zool (London) 175:107–149
Willumsen, N.J., Larsen, E.H. 1982. Time-and voltage-characteristics of a passive Cl− pathway in the isolated skin ofBufo bufo.Acta Physiol. Scand. 114:28A
Willumsen, N., Larsen, E.H. 1984. Passive chloride transport of the toad skin does not pass through granular cells.Acta Physiol. Scand. 120:46A
Willumsen, N.J., Larsen, E.H. 1985a. Membrane potentials and Cl− activity of the granulosum cells in toad skin in relation to activation/deactivation of the transepithelial voltage sensitive Cl− conductance.Acta Physiol. Scand. 124-Suppl. 542:156
Willumsen, N.J., Larsen, E.H. 1985b. Passive Cl− currents in toad skin: Potential dependence and relation to mitochondria-rich cell density.In: Transport Processes, Iono- and Osmoregulation. R. Gilles and M. Gilles-Baillien, editors. pp. 20–30. Springer, Berlin-Heidelberg
Zadunaisky, J.A., Candia, O.A., Chiarandini, D.J. 1963. The origin of the short-circuit current in the isolated skin of the South American frogLeptodactylus ocellatus.J. Gen. Physiol. 47:393–402
Zeiske, W., Van Driessche, W. 1979. Saturable K+ pathway across the outer border of frog skin (Rana temporaria): Kinetics and inhibition by Cs+ and other cations.J. Membrane Biol. 47:77–96
Zeuthen, T. 1984. The electrophysiology of ion and water transport in gallblader and other leaky epithelia. D. Sc. Thesis, University of Copenhagen
Zeuthen, T., Hiam, R.C., Silver, I.A. 1974. Recording of ion activities in the brain.In: Ion Selective Microelectrodes. H. Berman and N. Herbert, editors. pp. 145–156. Plenum London
Zylber, E.A., Rotunno, C.A., Cereijido, M. 1973. Ion and water balance in isolated epithelial cells of the abdominal skin of the frogLeptodactylus ocellatus.J. Membrane Biol. 13:199–216
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willumsen, N.J., Larsen, E.H. Membrane potentials and intracellular Cl− activity of toad skin epithelium in relation to activation and deactivation of the transepithelial Cl− conductance. J. Membrain Biol. 94, 173–190 (1986). https://doi.org/10.1007/BF01871197
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871197