Summary
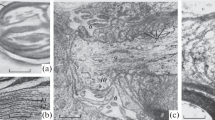
Ultrastructural changes in the nodal and paranodal regions of myelinated nerve fibres of frog optic nerves were studied during early stages of Wallerian degeneration. The earliest changes seen include retraction of paranodal loops of myelin from the axolemma and disconnection of paranodal myelin loops from myelin lamellae. These paranodal changes are asymmetric around the node and may be more advanced on either the proximal or distal side. Axoplasmic changes, including segregation of microtubules from neurofilaments, disorientation of microtubules and accumulation of abnormal organelles at nodes, appear shortly. In some axons the ‘undercoating’ along the widened nodal surfaces becomes patchy, and blebs appear in the nodal axolemma. In freeze-fracture replicas a mixture of particle clusters and particle-free areas appears in both E- and P-faces of the nodal axolemma. Blebs remain particle free. Initially, E-face particles remain segregated to the node and are present only at much lower concentrations in the demyelinated paranodal axolemma, suggesting that they are not freely mobile at this stage. Nodal E-face particles begin to decrease on day 5 associated with an increase in particles at the adjacent demyelinated paranode, and by day 11 the particle distribution is uniformly low over the entire extent of the nodal and demyelinated paranodal axolemma. If nodal E-face particles represent sodium channels, as has been proposed, the sequence of changes in Wallerian degeneration would be compatible with a gradual redistribution of nodal sodium channels into the demyelinated paranode.
Similar content being viewed by others
References
Abrahams, P. H., Day, A. &Allt, G. (1981) The node of Ranvier in early Wallerian degeneration: a freeze-fracture study.Acta neuropathologica 54, 95–100.
Armstrong, J. A. (1950) An experimental study of the visual pathways in a reptile (Lacerta vivipara).Journal of Anatomy 84, 146–67.
Ballin, R. H. M. &Thomas, P. K. (1969) Changes at the node of Ranvier during Wallerian degeneration: an electron microscope study.Acta neuropathologica 14, 237–49.
Berthold, C. H. (1978) Morphology of normal peripheral axons. InPhysiology and Pathobiology of Axons (edited byWaxman, S. G.), pp. 3–64. New York: Raven Press.
Bignami, A. &Ralston, H. J. (1969) The cellular reaction to Wallerian degeneration in the central nervous system of the cat.Brain Research 13, 444–61.
Branston, N. M. &Fleming, D. G. (1968) Efferent fibers in the frog nerve.Experimental Neurology 20, 611–23.
Bray, G. M., Rasminsky, M. &Aguayo, A. J. (1981) Interactions between axons and their sheath cells.Annual Review of Neuroscience 4, 127–62.
Causey, G. &Palmer, E. (1952) Early changes in degenerating mammalian nerves.Proceedings of the Royal Society of London, Series B 139, 597–609.
Cook, R. D., Ghetti, B. &Wiśniewski H. M. (1974) The pattern of Wallerian degeneration in the optic nerve of newborn kittens: an ultrastructural study.Brain Research 75, 261–75.
Crevel, H. V. &Verhaart, W. J. C. (1963) The rate of secondary degeneration in the CNS. II. The optic nerve of the cat.Journal of Anatomy 97, 451–61.
Daniel, P. M. &Strich, S. J. (1969) Histological observations on Wallerian degeneration in the spinal cord of the baboon (Papio papio).Acta neuropathologica 12, 314–28.
Elfvin, L. G. (1961) The ultrastructure of the nodes of Ranvier in cat sympathetic nerve fibers.Journal of Ultrastructure Research 5, 374–87.
Ellisman, M. H. (1977) High voltage electron microscopy of cortical specializations associated with membranes at nodes of Ranvier.Journal of Cell Biology 75, 108a.
Ewert, J. P. &Borchers, H. W. (1974) Inhibition of toad (Bufo bufo) retinal on-off and off-ganglion cells via active eye closing.Vision Research 14, 1275–6.
Fisch, U. (1980) Maximal nerve excitability testing vs. electroneuronography.Archives of Otolaryngology 106, 352–7.
Gilliat, R. W. &Hjorth, R. J. (1972) Nerve conduction during Wallerian degeneration in the baboon.Journal of Neurology, Neurosurgery and Psychiatry 35, 335–41.
Goldby, F. (1937) An experimental investigation of the cerebral hemisphere ofLacerta viridis.Journal of Anatomy 71, 332–55.
Hirano, A. &Dembitzer, H. M. (1967) A structural analysis of the myelin sheath in the central nervous system.Journal of Cell Biology 34, 555–67.
Kruger, L. &Maxwell, D. S. (1969) Wallerian degeneration in the optic nerve of a reptile: an electron microscopic study.American Journal of Anatomy 125, 247–70.
Lampert, P. W. &Cressman, M. R. (1966) Fine-structural changes of myelin sheaths after axonal degeneration in the spinal cord of rats.American Journal of Pathology 49, 1139–55.
Livingston, R. B., Pfenninger, K., Moor, H. &Akert, K. (1973) Specialized paranodal and interparanodal glial-axonal junctions in the peripheral and central nervous system: a freeze-fracture study.Brain Research 58, 1–24.
Luse, S. A. &McCaman, R. E. (1957) Electron microscopy and biochemistry of Wallerian degeneration in the optic and tibial nerves.American Journal of Pathology 33, 586.
Maturana, H. R. (1958) Efferent fibers in the optic nerve of a toad (Bufo bufo).Journal of Anatomy 92, 21–7.
Maturana, H. R. (1959) Number of fibers in the optic nerve and the number of ganglion cells in the retina of anurans.Nature 183, 1406–7.
McDonald, W. I. (1972) The time course of conduction failure during degeneration of a central tract.Experimental Brain Research 14, 550–6.
McDonald, W. I. (1976) Conduction in the optic nerve.Transactions of the Ophthalmological Society of the United Kingdom 96, 352–4.
Nathaniel, E. J. H. &Pease, D. C. (1963) Degenerative changes in rat dorsal roots during Wallerian degeneration.Journal of Ultrastructure Research 9, 511–32.
Neumann, E. (1868) Degeneration und regeneration nach nerven Durschschneidungen.Archiv für Heilkunde 9, 193–218.
Ohmi, S. (1961) Electron microscopic study on Wallerian degeneration of peripheral nerve.Zeitschrift für Zellforschung und mikroskopische Anatomie 54, 39–67.
Pecci Saavedra, J., Vaccarezza, O. L. &Mascitti, T. A. (1969) Degeneration in the parvocellular portion of the lateral geniculate nucleus of the cebus monkey. A light and electron microscope study.Zeitschrift für Zellforschung und mikroskopische Anatomie 93, 164–81.
Peters, A. (1966) The node of Ranvier in the central nervous system.Quarterly Journal of Experimental Physiology 51, 229–36.
Pilling, J. B. (1978) Nerve conduction during Wallerian degeneration in man (editorial).Muscle and Nerve 1, 81.
Rosenbluth, J. (1966) Redundant myelin sheaths and other ultrastructural features of the toad cerebellum.Journal of Cell Biology 28, 73–94.
Rosenbluth, J. (1976) Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain.Journal of Neurocytology 5, 731–45.
Rosenbluth, J. (1984) Membrane specializations at the nodes of Ranvier and paranodal and juxtaparanodal regions of myelinated central and peripheral nerve fibers. InThe Node of Ranvier (edited byZagoren, J. C. &Fedoroff, S.), pp. 31–67. Orlando: Academic Press.
Rosenbluth, J., Sumner, A. &Saida, T. (1981) Dedifferentiation of the axolemma associated with demyelination.Proceedings of 39th Annual Meeting, Electron Microscopy Society of America (edited byBailey, G. W.), pp. 496–7. Baton Rouge: Claitor's Pub. Division.
Rosenbluth, J., Tao-Cheng, J. H. &Blakemore, W. (1985) Dependence of axolemmal differentiation on contact with glial cells in chronically demyelinated lesions of the cat spinal cord.Brain Research 358, 287–302.
Scalia, F. &Teitelbaum, I. (1978) Absence of efferents to the retina in the frog and toad.Brain Research 153, 340–4.
Schnapp, B., Peracchia, C. &Mugnaini, E. (1976) The paranodal axo-glial junction in the central nervous system studied with thin sections and freeze-fracture.Neuroscience 1, 181–90.
Skoff, R. P. &Vaughn, J. E. (1970) An autoradiographic study of cellular proliferation in degenerating rat optic nerve.Journal of Comparative Neurology,141, 133–56.
Stelzner, D. J. &Strauss, J. A. (1986) A quantitative analysis of frog optic nerve regeneration: is retrograde ganglion cell death or collateral axonal loss related to selective reinnervation?Journal of Comparative Neurology,245, 83–106.
Tao-Cheng, J. H. &Rosenbluth, J. (1984) Extranodal particle accumulations in the axolemma of myelinated frog optic axons.Brain Research 308, 289–300.
Vaughn, J. E. &Pease, D. C. (1970) Electron microscopic studies of Wallerian degeneration in rat optic nerves. II. Astrocytes, oligodendrocytes and adventitial cells.Journal of Comparative Neurology 140, 207–26.
Waller, A. V. (1850) Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres.Philosophical Transactions of the Royal Society of London 140, 423–9.
Webb, W. W., Barak, L. S., Tank, D. W. &Wu, E. S. (1981) Molecular mobility on the cell surface.Biochemical Society Symposia 46, 191–205.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishise, J., Rosenbluth, J. Nodal and paranodal structural changes in frog optic nerve during early Wallerian degeneration. J Neurocytol 15, 657–670 (1986). https://doi.org/10.1007/BF01611864
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01611864