Abstract
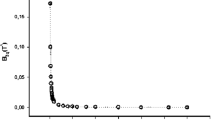
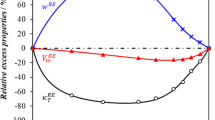
The speed of sound has been measured in the binary gaseous mixture (0.85CH4+0.15C3H8) along seven isotherms at temperatures between 225 and 375 K and at pressures up to 1.4 M Pa. From the measurements, second and third acoustic virial coefficients of the mixture were obtained. These results were analyzed together with values of the second and third acoustic virial coefficients of the two pure components to obtain a set of model intermolecular potential-energy functions for the methane-propane system. Nonpairwise additivity of the intermolecular forces was included in this analysis. Ordinary second and third interaction virial coefficients calculated from the model are reported, as are the second and third virial coefficients of the pure components. Gas densities calculated by means of these virial coefficients for the mixture (0.9298CH4+0.0702C3H8) were found to agree with experimental values at temperatures between 280 and 330 K to within 0.02% at pressures up to 3 MPa and to within 0.08% at 4MPa.
Similar content being viewed by others
References
K. E. Starling, M. Mannan, J. L. Savidge, S. Sadisvan, T. B. Reid Jr., K. Gangadhar, and M. A. Drass,Development o/ an Equation a/ State for computation o/ Supercompressibility Factors, Critical Flow Factors and Other Properties o/ Wet Sour Natural Gas, Synthetic Gases and Admixtures, Final Report (Gas Research Institute, Chicago, sept. 1937).
K. E. Starling and J. L. Savidge,Compressibility Factors of Natural Gas and Other Related Hydrocarbon Gases, AGA Transmission measurement committee report No. 3, 2nd ed. (American Gas Association, Arlington, VA, Nov. 1992).
M. Jaeschke, S. Audibert, P. van Caneghem, A. E. Humphreys, R. Jansseenvan Rosmalen, Q. Pellei, J. A. Schouten, and J. P. J. Michels,SPE Prod. Eng. 350 (1991 ).
J. P. M. Trusler and M. Zarari,J. Chem. Thermodyn. 24:973 (1992).
J. P. M. Trusler,J. Chem. Thermodyn. 26:751 (1994).
J. P. M. Trusler, W. A. Wakeham, and M. P. Zarari,Hikh. Temp. High Press. 25:293 (1993).
B. I. Lee and M. G. Kesler,AIChE J. 21:510 (1975).
M. P. Zarari, Ph.D. thesis (University of London, 1993).
J. P. M. Trusler,Physical Acoustics and Aletrology al Fluids (Adam Hilger, Bristol. 1991 ), P. 9.
G. C. Maitland and E. B. Smith.Chem. Phys. Lett. 22:443 (1973).
M. Frenkel, N. M. Gadather, K. N. Marsh, and R. C. Willhoit, eds.TRC Thermodynamic Tables: Hydrocarbons. vot. 1 (Thermodynamics Research center, TX, 1993).
K. T. Tang,Phys. Rer. 177:108 (1969).
M. Jaeschke and A. E. Humphreys,The GERG Databank o/ High Accurancy Comprcssibility Factor Measurements, (GERG, 1990), p. 196–198.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trusler, J.P.M., Wakeham, W.A. & Zarari, M.P. Second and third interaction virial coefficients of the (methane+propane) system determined from the speed of sound. Int J Thermophys 17, 35–42 (1996). https://doi.org/10.1007/BF01448207
Issue Date:
DOI: https://doi.org/10.1007/BF01448207