Abstract
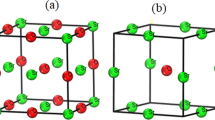
A self-consistent LAPW band structure calculation for TiO0.75 has been performed, assuming long-range order of vacancies on the oxygen sublattice. The calculation is based on a hypothetical model structure which can be described as Ti [4]3 Ti[6]O3□o, where □o denotes an oxygen vacancy. In the model structure two types of titanium atoms occur: Ti[4] atoms which have two vacancies as neighbours and are quadratically surrounded by four oxygen atoms, and Ti[6] atoms which are octahedrally surrounded by six oxygen atoms. The calculated density of states (DOS) and the local partial densities of states are compared with the respective values for stoichiometric TiO containing no vacancies. A characteristic difference is the appearance of two sharp peaks in the DOS curve below the Fermi energy which are caused by the vacancies. These “vacancy states” exhibit a considerable amount of charge in the vacancy muffin-tin sphere and are found to be derived from Ti 3d states extending into the vacancy sphere. The introduction of vacancies also leads to a lowering of the Fermi energy indicating a stabilizing effect. The bonding situation in TiO0.75 as compared to TiO as well as the changes in chemical bonding in the series TiC0.75−TiN0.75−TiO0.75 are discussed on the basis of electron density plots. The loss of Ti[4]−O bonds (as compared to TiO) is compensated by the formation of Ti[4]−□o−Ti[4] bonds across the vacancy and by an increase of the bond strength of the Ti[4]−O bonds. On the other hand, the Ti[4]−Ti[4] and particularly the Ti[4]−Ti[6] bonds are weakened by the introduction of vacancies.
Similar content being viewed by others
References
Murray, J.L., Wriedt, H.A.: Bull.Alloy Phase Diagr.8, 148 (1987)
Watanabe, D., Castles, J.R., Jostsons, A., Malin, A.S.: Acta Crystallogr.23, 307 (1967)
Hilti, E.: Naturwissenschaften55, 130 (1968)
Banus, M.D., Reed, T.B., Strauss, A.J.: Phys. Rev. B5, 2775 (1972)
Bilz, H.: Z. Physik153, 338 (1958)
Yamashita, J.: J. Phys. Soc. Jpn.18, 1010 (1963)
Honig, J.M., Wahnsiedler, W.E., Dimmock, J.O.: J. Solid State Chem.5, 452 (1972)
Ern, V., Switendick, A.C.: Phys. Rev.137, 202 (1965)
Mattheiss, L.F.: Phys. Rev. B5, 290 (1972)
Neckel, A., Rastl, P., Eibler, R., Weinberger, P., Schwarz, K.: J. Phys. C.: Solid State Phys.9 579 (1976)
Schoen, J.M., Denker, S.P.: Phys. Rev.184, 864 (1969)
Burdett, J.K., Hughbanks, T.: J Am. Chem. Soc.106 3101 (1984)
Schwarz, K.: Phys. Chem. Minerals14, 315 (1987)
Hörmandinger, G., Redinger, J., Weinberger, P., Hobiger, G., Herzig, P.: Solid State Commun.68, 467 (1988)
Redinger, J., Eibler, R., Herzig, P., Neckel, A., Podloucky, R., Wimmer, E.: J. Phys. Chem. Solids46, 383 (1985)
Redinger, J., Eibler, R., Herzig, P., Neckel, A., Podloucky, R., Wimmer, E.: J. Phys. Chem. Solids47, 387 (1986)
Herzig, P., Redinger, J., Eibler, R., Neckel, A.: J. Solid State Chem.70, 281 (1987)
Hobiger, G., Herzig, P., Eibler R., Schlapansky, F., Neckel, A.: J. Phys.: Condensed Matter (submitted for publication)
Andersen, O.K.: Phys. Rev. B12, 3060 (1975)
Koelling, D.D., Arbman, G.O.: J. Phys. F.: Met. Phys.5, 2041 (1975)
Hedin, L., Lundqvist, S.: J. Phys. Paris33, C3–73 (1972)
Lehmann, G., Taut, M.: Phys. Status Solidi (b)54, 469 (1972)
Neckel, A., Schwarz, K., Eibler, R., Weinberger, P., Rastl, P.: Ber. Bunsenges. Phys. Chem.79, 1053 (1975)
Author information
Authors and Affiliations
Additional information
Dedicated to Professor F. Kohler on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Schlapansky, F., Herzig, P., Eibler, R. et al. The influence of oxygen vacancies on the chemical bonding in titanium oxide. Z. Physik B - Condensed Matter 75, 187–195 (1989). https://doi.org/10.1007/BF01307999
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01307999