Abstract
The 18-electron rule and the corresponding methods for counting the total valence electrons of transition metal complexes are among the most useful basic tools in modern inorganic chemistry, particularly in its application to organometallic species. While in its simplest representation, the 18-electron rule is explained in that a closed, stable noble gas configuration of ns 2(n-1)d 10 np 6 is achieved with 18 valence electrons, this does not adequately explain the trends and exceptions seen in practice. As such, this report presents a deeper discussion of the 18-electron rule via molecular orbital models, stressing the roles of both σ- and π-bonding effects. This discussion thus aims to provide a better understanding of the relationship between electron count and stability, while also illustrating which factors can determine adherence (or not) to this commonly utilized rule. Lastly, the two common methods for electron counting (ionic and covalent models) are also presented with practical examples to provide the complete ability to apply the 18-electron rule.
Similar content being viewed by others
Introduction
The 18-electron rule and the corresponding methods for determining the total number of valence electrons in metal compounds, commonly referred to as ‘electron counting’, are among the most useful basic tools in modern inorganic chemistry, particularly in its application to organometallic species. Not only can it be a predictive tool for evaluating the stability of some inorganic complexes, but can also be used to predict potential formulas of stable compounds. In addition, changes in the electron count of metal complexes during chemical processes can be a critical factor in determining mechanistic details of reaction pathways.
Historian William B. Jensen has traced the origins of the modern 18-electron rule to 1921 [1], when Irving Langmuir (1881–1957) [2] of the General Electric Company (Fig. 1) presented new models of valence, in an attempt to extend the Lewis static-atom model to cases not adequately explained by “octet theory” (i.e., the modern “octet rule”) [3]. In this early work, Langmuir derived an equation which related the number of shared (i.e., bonding) electrons or the covalence (v c) of a given atom in a compound to the difference between the number of valence electrons (e) in the isolated neutral atom and the number of valence electrons (s) after formation of the compound:
For any complete compound, s = 2, 8, 18, or 32. As such, the value of s corresponds to complete closed shell electron configurations and would correspond to the normal 8 for compounds of p-block elements as illustrated by the octet rule. Extending this model beyond the octet, however, leads to s = 18 for compounds of d-block elements and s = 32 for compounds f-block elements. Thus, for the d-block elements, a simple algebraic rearrangement leads to the relationship
which states that the sum of the metal valence electrons and the bonding electrons contributed by the ligands equals a total of 18 valence electrons. Langmuir then illustrated how the metal carbonyl compounds Ni(CO)4, Fe(CO)5, and Mo(CO)6 all exhibited structures consistent with those predicted by this relationship [3, 4].
An alternative electron count model, known as the effective atomic number (EAN) rule, was introduced in 1927 by Nevil Sidgwick (1873–1952) [5] of Oxford University [1, 4, 6, 7]. Like the Langmuir model, stability was assumed to be dependent on obtaining a noble gas configuration for the central atom. In contrast, however, the EAN of a compound was analogous to the atomic number of an atom and thus focused not just on the number of valence-shell electrons, but the total electron count of the compound’s central atom. Thus, for transition metals, the EAN rule was said to be obeyed when d-block compounds obtained EAN values equal to the total electron configurations of noble gases Kr, Xe, and Rn (i.e. 36, 54, or 86) [7]. For the transition metals, attainment of an 18-electron valence configuration would therefore be equivalent to attaining the total electron count or EAN of the nearest noble gas [1].
As detailed by Jensen [1], there was a reversion to the earlier approach of Langmuir by the late 1960s. This was most likely due to the fact that Sidgwick’s model includes both the valence and core electrons, thus resulting in a different electron count in relation to stability for each row of the d-block. In contrast, Langmuir’s procedure, like the octet rule, has the advantage of using a single valence electron count for the three rows of the transition metals, thus eliminating the need to remember a different EAN value for each of the corresponding noble gases [1, 6]. Another factor contributing to this shift in approach could also have been a paper by Craig and Doggett published in 1963 that tried to present a theoretical basis for Sidgwick’s model, which they referred to as the “rare-gas rule” [8]. In this treatment, they began with the full electron count model, but quickly refocused the discussion to present the work relative to the 18 valence electrons of the d-block elements.
Overall, the common aspects of the Langmuir and Sidgwick models relating to the d-block can be combined to make the general statement that when the metal of a complex obtains an outershell configuration of ns 2(n-1)d 10 np 6, the valence orbitals contain 18 electrons and a closed, stable configuration is achieved. This basic relationship is commonly referred to as the “18-electron rule”, which has become a guiding principle of inorganic chemistry, particularly in organometallic chemistry [8–10]. It should be pointed out that some use the terms “18-electron rule” and “effective atomic number (or EAN) rule” interchangeably [9, 11–13]. However, as the discussion above correctly demonstrates, the 18-electron rule is consistent with the EAN rule and may thus be considered a component or outcome of Sidgwick’s model, but these terms are not synonymous. In addition to the different emphasis on valence versus total electrons, the 18-electron rule is strictly limited to the d-block elements, while the EAN rule can be applied to all elements of the periodic table.
Much like the more common octet rule, the 18-electron rule is not always strictly obeyed and is subject to a number of apparent exceptions [10]. Thus, while this tool is extremely useful in predicting stability, examples of stable metal complexes with more or less than 18 valence electrons are also fairly common [1, 6, 9]. As such, the goal of this report is to present a deeper discussion of the 18-electron rule to better understand the relationship between electron count and stability, while also illustrating which factors can determine adherence (or not) to this commonly utilized rule.
18-Electron rule in terms of molecular orbital models, part I: simple view
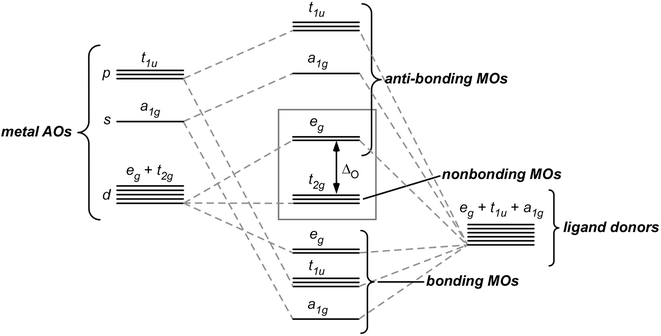
Further insight into the connection between the stability of metal compounds and the 18-electron rule can be gained by addressing this relationship in terms of the molecular orbital (MO) description of bonding in metal complexes. As a starting point, let us consider just the simple σ bonding between a metal and six identical σ-donor ligands to generate an octahedral metal complex. Linear combination of the ligand orbitals with the s, p, and d atomic orbitals of the metal results in the formation of six bonding MOs (a 1g , t 1u , and e g ) and six corresponding antibonding MOs, as illustrated in Fig. 2. As there are a total of nine metal-based atomic orbitals and only six ligand-based donor orbitals, this results in three d orbitals remaining as non-bonding t 2g MOs.
Nevil Sidgwick (1873–1952) [Reproduced from [5]; Courtesy of JSTOR]
For such an octahedral complex, the most stable arrangement will be that in which all of the bonding MOs (a 1g , t 1u , and e g ) are fully occupied and the anti-bonding MOs are empty. Occupation of the six bonding MOs, as well as the three nonbonding MOs, would thus require 18 electrons, as predicted by the 18-electron rule [4, 11]. As such, complexes will therefore tend to adhere to the rule if the energetic separation between the non-bonding t 2g and anti-bonding e g MOs (commonly referred to as ΔO, Fig. 3) is large [6, 12]. Here, as ΔO increases, the e g MOs are destabilized and ultimately reside at higher energies, thus making occupation of these anti-bonding orbitals unfavorable.
The value of ΔO depends on both the central metal and the specific ligands involved. In terms of the metal, ΔO tends to increase down any particular periodic group (i.e. 3d < 4d < 5d) and for the same metal, it tends to increase with the metal charge (i.e. M2+ < M3+ < M4+) [9, 14, 15]. As such, complexes of second and third row transition metals are typically not found to have more than 18 electrons [4].
The effect of the associated ligands is represented by the spectrochemical series (Fig. 4) [14–17], which orders the ligands from the smallest value of ΔO to the largest. In the most general sense, the order within the spectrochemical series is indicative of the ligand σ-donor strength. However, ligands low on the spectrochemical series also tend to exhibit π-donor characteristics, while those high on the series are typically strong π-accepting ligands. While these σ and π effects are typically additive, the π effects can sometimes play a more critical role than the simple σ-donor strength. The ligands within the series are generally ordered according to the periodic group of the ligand donor atom. As such, group 17 (i.e., the halogens) are low on the series, while group 14 are high on the spectrochemical series. Groups 15 and 16 then fall between these two extremes, as can be seen in Fig. 4 [14].
Although the factors discussed above are a good start to understanding the working parameters of the 18-electron rule, it does not adequately explain the various limitations of this common rule. While not a complete list, the most common limitations can be given as:
-
1.
This rule works best for low-valent (oxidation state ≤ 2) metal complexes, but many high valent metal complexes also work.
-
2.
This rule works best for complexes containing π-acceptor ligands.
-
3.
Square planar complexes are generally stable as 16-electron species.
-
4.
There are always exceptions to the rule.
To understand the basis of these common limitations, it will be necessary to consider a number of additional factors.
18-Electron rule in terms of molecular orbital models, part II: more detailed view
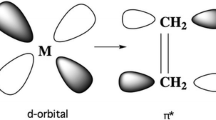
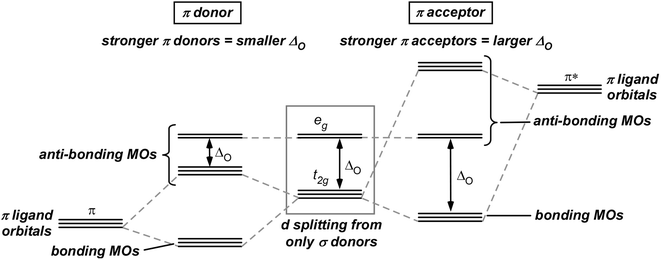
In the initial treatment above, only σ donor interactions were considered and any potential π interactions were not taken into account. To deepen our insight into the factors that contribute to the 18-electron rule, it is now required to complicate our bonding picture by including any potential π-donor or π-acceptor contributions from the organic ligands. As outlined in Fig. 5, the initial splitting of the d orbitals from the σ donors (i.e., the boxed orbitals in Figs. 3, 5) is further modified via interactions between π ligands and the metal t 2g orbitals, which are formally nonbonding in terms of the previous σ interactions. In the case of π-donor contributions, the ligand π-orbitals are filled and lower in energy than the metal t 2g . Mixing of these orbitals with the metal t 2g orbitals thus results in production of π-bonding metal–ligand MOs, while the t 2g orbitals take on π* anti-bonding character, resulting in their destabilization and a decrease in ΔO (Fig. 5) [12, 16, 18].
Things are a bit different in the case of π-acceptor contributions. In this case, the ligand orbitals interacting with the metal are empty π*-orbitals which are high in energy (Fig. 5). As such, mixing of these high-lying orbitals with the metal t 2g orbitals results in stabilization of the metal orbitals, that leads to an increase in the value of ΔO. Here, the stabilized t 2g orbitals are now formally π-bonding in nature, while the e g orbitals retain their σ* anti-bonding character [12, 16, 18, 19].
Transition metal complexes can be divided into three groups [9, 18], which will be referred to here as Class I, Class II, and Class III. The first of these groups, Class I, are complexes with a relatively small ΔO and generally consist of first row transition metals with ligands low on the spectrochemical series (i.e., weak σ donors or π donors). If the bonding in these complexes is only weak σ bonding, then the metal t 2g orbitals are formally nonbonding and can thus be either empty or filled without negatively affecting the extent of metal–ligand bonding. Here, the relatively weak σ bonding also results in the e g orbitals being low in energy and only weakly anti-bonding. As a result, the occupation of these MOs can also occur with little negative effect. Ligands with π-donor character, however, contribute to π bonding which results in the t 2g orbitals taking on anti-bonding character as shown in Fig. 5, and the occupation of these MOs is then not favored. It should be pointed out that strong π-donor ligands not only result in the t 2g becoming anti-bonding, but also generate complementary filled π-donor bonding orbitals localized largely on the ligands. In this case, occupation of these π-bonding orbitals while keeping the t 2g orbitals unoccupied maintains an 18 electron count [4]. For the most part, however, these complexes typically do not exhibit π-bonding of this magnitude and σ-bonding dominates the characteristics of these metal compounds. As such, the 18-electron rule has no special significance for the bulk of this class of complexes [12] and stable Class I species are possible with electron counts of 12–22 electrons depending on the extent that the t 2g and e g orbitals are occupied [6, 9, 18].
Class II complexes have a larger ΔO and generally contain second and third row transition metals (especially in higher oxidation states) with σ ligands intermediate to high on the spectrochemical series. For this class, there is intermediate to strong σ bonding, but no π bonding. As such, the t 2g orbitals are still nonbonding and can thus be either empty or filled. The relatively strong σ bonding, however, raises the energy of the e g orbitals and strengthens their anti-bonding nature, therefore making the occupation of these MOs disfavored. As a result, stable Class II complexes are possible with electron counts of 12–18 electrons, depending on the occupation of the t 2g orbitals [6, 9, 18]. Class II complexes can follow the 18-electron rule, but a fair number do not.
The final group, Class III, is made up of complexes exhibiting the largest ΔO. These complexes generally consist of low oxidation state metals with ligands high on the spectrochemical series, particularly π-acceptor ligands. The metal–ligand interactions here constitute intermediate to strong σ bonding, as well as significant π back-bonding into the empty π* orbitals of any π-acceptor ligands. As such, the t 2g orbitals now have π-bonding character and need to be filled for complex stability. In addition, the e g orbitals are still strongly anti-bonding and thus, the occupation of these MOs is again not favored. As a result, stable Class III complexes will always have the t 2g orbitals occupied and the e g orbitals empty, resulting in 18 valence electrons and strict adherence to the 18-electron rule [9, 18]. As almost all organometallic compounds of the d-block elements belong to Class III, the 18-electron rule becomes a very powerful and reliable predictive tool for organometallic chemistry.
Other compound geometries
Although the argument used above was developed using the example of an octahedral complex, precisely similar results are obtained for other coordination numbers. Generally it can be said that if the coordination number (CN) is greater than four, then [CN-4] d orbitals are required in addition to the metal s and p orbitals to form MOs with the corresponding ligand σ orbitals. As a result, this will result in [CN-4] low-lying anti-bonding orbitals and [9-CN] nonbonding orbitals. The energetic separation between these two general sets of orbitals will largely depend on the strength of the σ donors as discussed above [9].
This general relationship is illustrated for CN = 6 for the octahedral geometry given in Fig. 3, but can also be shown for CN = 5 as given for the trigonal bipyramidal geometry shown in Fig. 6. As with the octahedral example, filling the bonding and nonbonding MOs here would correspond to 18 electrons and be consistent with the 18-electron rule. Effects on adherence to this rule based on σ-donor strength and any contribution of π-bonding would again mirror those previously discussed for the octahedral case [4, 19].
The situation is more complicated for tetrahedral complexes (CN = 4) [6, 9, 19]. In this case, bonding with the four σ-donors can be proposed using either d 3 s or sp 3 sets of metal orbitals, although in practice a combination of both sets are used and the t 2 orbitals of the complex will involve both d and p orbitals from the metal (Fig. 7) [9]. As the resulting four bonding MOs can only accommodate a total of eight electrons, the remaining ten electrons must occupy the e and t 2 sets of d orbitals, which are formally nonbonding and at least partially antibonding [6]. The reduced number of ligands in comparison to the previous octahedral case results in a smaller energetic separation between these two sets of orbitals (Δ T ) and thus, there is little barrier to population of the t 2 orbitals [9].
Because of these factors, the 18-electron rule is only obeyed in tetrahedral complexes of π-acceptor ligands. In these cases, both the e and t 2 sets of d orbitals donate electrons to the empty π* orbitals of the ligands, resulting in both sets of orbitals becoming bonding in nature. In addition, MO calculations have shown that these π-bonding interactions contribute more to the metal–ligand bonding than the corresponding σ-donation from the ligand [19, 20].
As stated in the general limitations above, the one major geometry that does not obey the 18-electron rule is that of four-coordinate, square planar complexes [4, 6, 9, 19]. As shown in Fig. 8, the metal-based orbitals are split into four sets, with the majority being nonbonding or having some partial bonding character. The remaining orbital (corresponding to the \(d_{{x^{ 2} - y^{ 2} }}\)) is anti-bonding and is fairly high in energy [19]. For these complexes, filling the four low-lying σ bonding MOs requires 8 electrons and population of the metal-based bonding and nonbonding orbitals requires another 8 electrons. Any additional electrons would then populate the \(d_{{x^{ 2} - y^{ 2} }}\) (b 1g ) σ* orbital, resulting in a decrease in stability. As such, a total of 16 electrons represent the maximum population for a stable square planar complex [9, 19]. This is sometimes referred to as the “16-electron rule” [6] or added to the 18-electron rule to become the “16 and 18 electron rule” [10], but most often is just recognized as the most consistent exception to the 18-electron rule.
Electron counting methods
By counting the number of valence electrons surrounding a metal in a particular complex formula, it is possible not only to predict whether the complex should be stable, but in some cases give details concerning structural aspects of the complex (i.e., ligand binding modes, the presence of metal–metal bonds, etc.) [6]. Of course, as previously noted, while the electron count can be extremely useful in predicting stability, examples of stable metal complexes with more or less than 18 valence electrons are also fairly common [1, 6, 9]. Nevertheless, the ability to correctly determine the number of valence electrons from the complex formula is a critical step in the application of the 18-electron rule.
There are two methods for electron counting, the ionic model and the covalent model, each with its own advantages and limitations. Both models include the total valence electrons of the metal and those donated by the ligands, but differ in the way the division of electrons between the metal and the ligands are viewed. Either method can be used successfully and should provide the same answer, providing that care is taken not to mix aspects of the two models.
In the ionic model, the metal is treated as a cationic center and the ligands carry the charge associated with their non-coordinated state. As a consequence, this model requires that we correctly assign the metal a formal oxidation state. This in turn requires that we also know the formal charge on all the corresponding ligands. However, this model has the advantage that it treats most metal–ligand bonds as coordinate covalent (or dative) bonds which means each bond donates two electrons to the total electron count. The formal charge and corresponding electrons donating by a significant number of common ligands under the ionic model are given in Table 1.
In contrast to the ionic model, the covalent model treats the metal as a neutral center and all ligands are likewise treated as neutral species. While this does not affect the way that neutral ligands are counted, ligands that would be typically viewed as charged are now treated as radical species for the electron count. This model removes the need to determine the formal oxidation state of the metal, but is somewhat awkward for anionic ligands such as the halides. However, this model is much more realistic for many organometallic ligands, such as the simple alkyls. The corresponding electrons donated under the covalent model are given for all common ligands in Table 1.
The application of both models is illustrated via two examples given in Fig. 9. The first example, [RhCl2(bpy)2]+, represents a typical coordination complex including 2,2′-bipyridine (bpy) and chloride ligands. Under the ionic model, the combination of the overall 1+ charge and the two anionic ligands allows us to assign for formal 3+ oxidation state to the rhodium center. As such, this d 6 metal contributes six electrons, while each bpy and chloride ligand contributes four and two electrons, respectively, the sum of which gives the expected 18 electrons of a stable species.
Under the covalent model, the rhodium of the same complex is treated as Rh(0) and is thus a d 9 metal, contributing nine electrons to the electron count. As bpy is a neutral ligand, it is treated the same under both models and is thus a four-electron donor as before. Under the covalent model, however, the anionic chloride ligand is treated as a neutral radical and is thus a one-electron donor. The sum thus comes to 19 electrons, but as all components have been treated as neutral species, it is necessary to subtract one electron to account for the overall positive charge of the cationic complex. Thus, the total electron count comes to 18 electrons and this is in complete agreement with the count from the ionic model.
In the second example, [Ni(Et)2(CO)2], represents a typical organometallic complex including both alkyl (ethyl) and carbonyl ligands. Under the ionic model, the alkyl ligands are treated as anionic ligands and thus the nickel center is assigned a formal 2+ oxidation state to give an overall neutral complex. As such, this d 8 metal contributes eight electrons, while both the alkyl and carbonyl ligands contribute two electrons each, the sum of which gives the expected 16 electrons for a square planar species.
Under the covalent model, the nickel center is given a formal charge of zero and is thus a d 10 metal, contributing ten electrons to the electron count. The neutral carbonyl ligand is treated as before and is therefore still a two-electron donor. Under the covalent model, however, the ethyl ligands are now neutral radicals and are thus one-electron donors. As a result, the total electron count again comes to 16 electrons and is in complete agreement with the count from the ionic model. While the first example is more simply treated using the ionic model, this second example is more straight-forward under the covalent model. As such, both models have their benefits providing each are used independently.
Some additional structural factors that need to be considered are cases of bridging ligands and metal–metal bonding, both of which are somewhat common in organometallic species. A common class of bridging ligands are anionic species containing multiple lone pairs on the donor atom (halogens, RS−, RO−, R2P−, etc.). In this case, the ligand is able to utilize two different lone pairs to simultaneously donate to two separate metal centers. For such ligands, the first bond is treated exactly the same as a terminal anionic ligand and is thus a two-electron donor under the ionic model and a one-electron donor for the covalent model. As the second metal–ligand bond is via a full lone-pair under either model, this is treated the same as a neutral ligand and is thus a consistent two-electron donor to the second metal.
Two additional types of bridging ligands are bridging hydrides and bridging carbonyls, both of which only have a single lone-pair to donate. In the more simplistic case of the carbonyl, the donated electrons are split evenly between the two metal centers and this ligand is thus a one-electron donor to each metal under both models. The treatment of bridging hydrides, however, is a bit more complicated. Here the first bond is treated in the same way as a terminal hydride and is thus either a one- or two-electron donor. The second bond, however, is typically described as a donation of the first σ metal–ligand bond to the second metal. As such, it is treated as a two-electron donor to the second metal. Of course, this means that we are including the same two hydride-electrons in the electron count of both metals. Illustrations of electron counting examples that include all three types of bridging ligands are given in Fig. 10.
Finally, metal–metal bonds are treated as classically covalent interactions under both models and thus always contribute one electron to the total electron count. In this case, the donated electron comes from the valence electrons of the second metal center, which means that the electron is technically included in the electron count for both metals. Examples of electron counting that include metal–metal bonds are given in Fig. 10.
Conclusion
Adherence to the 18-electron count is strongest for metal complexes that include strong π-bonding. As such examples most commonly occur with π-acceptor ligands, strong π-bonding interactions require low-valent metals to provide sufficient electron density on the metal for back-donation to the empty ligand π* orbitals. Metal complexes with strong σ-donors, but no π-bonding can follow the 18-electron rule, but such complexes can exhibit stability with fewer than 18 valence electrons. While these relationships are not limited to octahedral geometries and hold true for many other common metal geometries, four-coordinate square planar complexes do not follow the 18-electron rule and are found to be stable with only 16 electrons. Lastly, proficient application of the 18-electron rule requires the ability to correctly count the total valence electrons for a given metal complex. This can be accomplished using one of two electron counting models (ionic or covalent), providing the chosen model is utilized exclusively.
References
Jensen WB (2005) The origin of the 18-electron rule. J Chem Educ 82:28
Rideal E (1959) Langmuir memorial lecture. Proc Chem Soc 1959:80
Langmuir I (1921) Types of Valence. Science 54:59–67
Mingos DMP (2004) Complementary spherical electron density model and its implications for the 18 electron rule. J Organomet Chem 689:4420–4436
Tizard HT (1954) Nevil Vincent Sidgwick 1873–1952. Obit Not Fellows R Soc 9:236
Huheey JE, Keiter EA, Keiter RL (1993) Inorganic chemistry, principles of structure and reactivity, 4th edn. HarperCollins, New York, pp 624–630
Sidgwick NV (1927) The electronic theory of valency. Clarendon Press, Oxford, pp 163–184
Craig DP, Doggett G (1963) Theoretical basis of the “rare-gas rule”. J Chem Soc 1963:4189–4198
Mitchell PR, Parish RV (1969) The eighteen-electron rule. J Chem Educ 46:811–814
Tolman CA (1972) The 16 and 18 electron rule in organometallic chemistry and homogeneous catalysis. Chem Soc Rev 1:337–353
Collman JP, Hegedus LS, Norton JR, Finke RG (1987) Principles and applications of organotransition metal chemistry. University Science Books, Mill Valley, pp 22–34
Powell P (1988) Principles of organometallic chemistry, 2nd edn. Chapman and Hall, New York, pp 148–152
Mingos DMP (1998) Essential trends in inorganic chemistry. Oxford University Press, Oxford, pp 297–309
Wulfsberg G (2000) Inorganic Chemistry. University Science Books, Sausalito, pp 367–374
Huheey JE, Keiter EA, Keiter RL (1993) Inorganic chemistry, principles of structure and reactivity, 4th edn. HarperCollins, New York, pp 404–405
Lever ABP (1968) Inorganic electronic spectroscopy. Elsevier Publishing Co., Amsterdam, pp 194–204
Osborn JA, Gillard RD, Wilkinson G (1964) J Chem Soc 1964:3168–3173
Porterfield WW (1993) Inorganic chemistry, a unified approach, 2nd edn. Academic Press Inc, San Diego, pp 604–609
Wulfsberg G (2000) Inorganic chemistry. University Science Books, Sausalito, pp 532–534
Bauschlicher CW, Bagus PS (1984) The metal-carbonyl bond in Ni(CO)4 and Fe(CO)5: a clear-cut analysis. J Chem Phys 81:5889–5898
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasmussen, S.C. The 18-electron rule and electron counting in transition metal compounds: theory and application. ChemTexts 1, 10 (2015). https://doi.org/10.1007/s40828-015-0010-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40828-015-0010-4