Summary
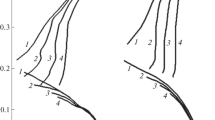
The electrochemical behaviour of Fe and C-steel samples in oxalic acid solutions was studied by the use of cyclic voltammetry. Two peaks were observed; the first one was the anodic peak and the second one an unexpected reductive dissolution peak which could be observed in the cathodic branch of cyclic voltammograms of all electrodes studied. The carbon content was found to increase the active dissolution of steels and to decrease their tendency towards passivation. The inhibitive efficiency of phenyl phthalimide derivatives on the active dissolution of Fe and steel samples in 0.1M oxalic acid were investigated.
Zusammenfassung
Das elektrochemische Verhalten von Eisen und C-Stählen in Oxalsäurelösungen wurde mit Hilfe cyclischer Voltammetrie studiert. Dabei treten zwei Signale auf: das anodische Signal sowie ein unerwartetes reduktives Lösungssignal. Letzteres wurde im kathodischen Zweig der cyclischen Voltammogramme aller untersuchten Elektroden gefunden. Der Kohlenstoffgehalt erhöht die aktive Auflösung von Stählen und erniedrigt ihre Tendenz zur Passivierung. Die Inhibitionseffizienz von Phenylphthalimiden für die aktive Auflösung von Eisen und Stahlproben in 0.1M Oxalsäure wurde untersucht.
Similar content being viewed by others
References
Krapivkina T. A., Marchenko T. G., Martynovai I. N. (1985) Zasch Metal21: 258
Shapovalov E. T., Kazakova G. V. (1983) Zasch Metal20: 929
Ivascann S., Georgescu O (1979) Rev. Chim.30: 360
Ivascann S., Georgescu O (1979) Rev. Chim.30: 568
Schmitt G., Bedbur K. (1984) Proc 9th Int. Congr. on “Metallic Corrosion.” Toronto, Canda, p 112
Granese S. L. (1987) Proc 10th Int. Congr. “Metallic corrosion” Madras, India, p 2733
Granese S. L. (1988) Corrosion44: 332
Granese S. L., Degonzalez C. O., Rosales B. (1988) Quimindustria La Habana, Cuba88: 176
Lorenz W. J., Ltibert F., Miyeshi Y., Eichkorn C. (1972) Proc. 5th Int. Cong. Metallic Corros. (Toryo), p 74
Abd El-Wahab F. M., Shams El-Din A. M. (1978) Br. Corrosion J.13: 39
Vetter K. V. (1961) Elektrochem. Kinetik. Springer, Berlin
Markoric T. (1964) Werkst. Korros.15: 543
Sato N., Kuda K., Noda T (1971) Electrochem. Acta.16: 1909
Scully J. S. (1975) The Fundamental of Corrosion, 2nd edn. Pergamon Press, p 118
Cohen M., Oswin H. (1957) J. Electrochem. Soc.9: 104
Lakhtin Y. (1971) Engineering Physical Metallurgy. Mir publishers, Moscow
Uhlig H. H. (1963) Corrosion and Corrosion Control, 2nd edn. John Wiley, p. 30
Uehara J., Aramaki K. (1991) J. Electrochem. Soc.138(11): 345
Khamis E., Bellucci F., Latanision R. M., El-Ashry E.SH. (1991) Corrosion47(9): 667
Masfeld E. (1987) Corrosion Mechanisms. Marcel Dekker, p 119
Shams El-Din A. M., Abd El-Haleem S. M. (1973) Werkstoffe Korros.24: 389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abdallah, M., Megahed, H.E. Cyclic voltammograms of iron and C-steels in oxalic acid solutions and investigation of the effect of phenyl phthalimide as corrosion inhibitors. Monatsh Chem 126, 519–527 (1995). https://doi.org/10.1007/BF00807424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807424