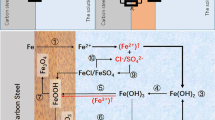
Abstract
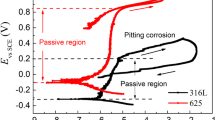
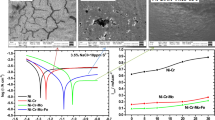
In this research, the effects of Ti addition, increased Al content (x = 12, 19 and 28 wt%) and pH of the corrosive solution on the corrosion behaviour of Fe, Fe–12Al, Fe–19Al, Fe–28Al and Fe–7.7Al–8.5Ti alloys were electrochemically studied through the potentiodynamic polarization (PDP), open-circuit potential (OCP) and electrochemical impedance spectroscopy (EIS) measurements. In all test solutions, the OCP reported generally that the open-circuit potential shift is positive compared to the Fe-alloy. The PDP results confirmed that the investigated alloys record a decrease in the corrosion rate in the following order: Fe–7.7Al–8.5Ti < Fe–xAl < Fe. Also, the corrosion current density (icorr) decreases with increasing the Al content. The experimental impedance data clearly confirmed that, increased Al content in the binary Fe–xAl alloys increases the corrosion resistance; moreover, ternary Fe–Al–Ti was superior to Fe–xAl against corrosion where more thicker and resistant passive layer is formed on the Fe–Al–Ti alloy surface. For all alloys, the corrosion resistance reaches maximum passivation in neutral rather than acidic or basic solution. The charge-transfer resistance, Rct, was compared with the polarization resistance, Rp, for accurate analysis of the EIS and PDP results. The surface analysis (SEM/EDAX) showed the participation of the different alloying elements in the protection according to the alloy constituents. It turned out that presence of Al and Ti increase the corrosion resistance. For industrial applications, the results lead to the recommendation of the Fe–7.7Al–8.5Ti alloy in the industrial chemical processes which require high corrosion resistance as that of halides resistance.













Similar content being viewed by others
References
Sherif E-SM, Almajid AA, Khalil KA, Junaedi H, Latief F (2013) Electrochemical studies on the corrosion behavior of API X65 pipeline steel in chloride solutions. Int J Electrochem Sci 8:9360–9370
Zamanzade M, Barnoush A, Motz C (2016) A review on the properties of iron aluminide intermetallics. Crystals 6:10
Ruan Y, Mohajerani A, Dao M (2016) Microstructural and mechanical-property manipulation through rapid dendrite growth and undercooling in an Fe-based multinary alloy. Sci Rep 6:31684
Brotzu A, Felli F, Marra F, Pilone D, Pulci G (2018) Mechanical properties of a TiAl-based alloy at room and high temperatures. Mater Sci Technol 34:1847–1853
Wen Y, Wang L, Liu H, Song L (2017) Ab initio study of the elastic and mechanical properties of B19 TiAl. Crystals 7:39
Chanbi D, Ogam E, Amara S, Fellah Z (2018) Synthesis and mechanical characterization of binary and ternary intermetallic alloys based on Fe–Ti–Al by resonant ultrasound vibrational methods. Materials 11:746
Nazarova T, Imayev V, Imayev R, Fecht H-J (2017) Study of microstructure and mechanical properties of Ti–45Al–(Fe, Nb)(at.%) alloys. Intermetallics 82:26–31
Kutz TN, Zander D (2017) The influence of chromium on the passivation of Fe3Al iron aluminides, investigated via potentiodynamic polarization in 0.25 M H2SO4. Corrosion 73:648–654
Karapanou E, Lekatou A, Sfikas A, Georgatis E, Lentzaris K, Karantzalis A (2017) Vacuum arc melting processed Fe-Al matrix based intermetallic composites, reinforced with VC phases: assessment of microstructure, sliding wear and aqueous corrosion response. Mater Sci Eng Adv Res. https://doi.org/10.24218/msear.2017.1S
Rosalbino F, Carlini R, Zanicchi G, Scavino G (2016) Effect of copper alloying addition on the electrochemical corrosion behaviour of Fe3Al intermetallic in sulphuric acid solution. Mater Corros 67:1042–1048
Peng J, Moszner F, Rechmann J, Vogel D, Palm M, Rohwerder M (2019) Influence of Al content and pre-oxidation on the aqueous corrosion resistance of binary Fe–Al alloys in sulphuric acid. Corros Sci 149:123–132
Romo L, Gonzalez-Rodriguez J, Porcayo-Calderon J, Guardian R, Salinas-Bravo V (2015) A study on the effect of Co, Cr and Ti on the corrosion of FE40AL intermetallic in molten NaCl–KCl mixture. Intermetallics 67:156–165
Bodunrin MO, Chown LH, van der Merwe JW, Alaneme KK (2019) Corrosion behaviour of low-cost Ti–4.5 Al–x V–y Fe alloys in sodium chloride and sulphuric acid solutions. Corros Eng Sci Technol 54:637–648
Bodunrin MO, Chown LH, van der Merwe JW, Alaneme KK (2018) Corrosion behaviour of Ti–Al–xV–yFe experimental alloys in 3.5 wt% NaCl and 3.5 M H2SO4. Mater Corros 69:770–780
Bodunrin MO (2018) Hot deformation and corrosion behaviour of low-cost α + β titanium alloys with aluminium, vanadium and iron addictions. Dissertation, University of Witwatersrand, Johannesburg
Zhang LC, Chen LY (2019) A review on biomedical titanium alloys: recent progress and prospect. Adv Eng Mater 21:1801215
Lu J, Zhao Y, Niu H, Zhang Y, Du Y, Zhang W, Huo W (2016) Electrochemical corrosion behavior and elasticity properties of Ti–6Al–xFe alloys for biomedical applications. Mater Sci Eng C 62:36–44
Dai N, Zhang L-C, Zhang J, Chen Q, Wu M (2016) Corrosion behavior of selective laser melted Ti–6Al–4V alloy in NaCl solution. Corros Sci 102:484–489
Khan MM, Shabib I, Haider W (2019) A combinatorially developed Zr–Ti–Fe–Al metallic glass with outstanding corrosion resistance for implantable medical devices. Scripta Mater 162:223–229
Qiu Y, Thomas S, Fabijanic D, Barlow A, Fraser H, Birbilis N (2019) Microstructural evolution, electrochemical and corrosion properties of AlxCoCrFeNiTiy high entropy alloys. Mater Des 170:107698
Yin Y, Pan C, Zhang R, Zhao C, Qu Y (2018) The effect of Ti addition on the microstructure and properties of high chromium iron-based coatings. J Alloys Compd 765:782–790
Sandlöbes S, Korte-Kerzel S, Raabe D (2019) On the influence of the heat treatment on microstructure formation and mechanical properties of near-α Ti–Fe alloys. Mater Sci Eng A 748:301–312
Candan S, Cim S, Candan E (2019) Effectiveness of Ti micro-alloying for the suppression of Fe impurities in AZ91 Mg alloys and associated corrosion properties. Mater Test 61:1165–1170
Zhao Y, Wang M, Cui H, Zhao Y, Song X, Zeng Y, Gao X, Lu F, Wang C, Song Q (2019) Effects of Ti-to-Al ratios on the phases, microstructures, mechanical properties, and corrosion resistance of Al2–xCoCrFeNiTix high-entropy alloys. J Alloys Compd 805:585–596
Bolzoni L, Ruiz-Navas EM, Gordo E (2016) Understanding the properties of low-cost iron-containing powder metallurgy titanium alloys. Mater Des 110:317–323
Flores-Chan J, Torres-Islas A, Patiño-Carachure C, Rosas G, Espinosa-Medina M (2016) Corrosion study of Al–Fe (20 wt%) alloy in seawater alkaline solutions. Int J Electrochem Sci 11:7359–7369
Huape-Padilla E, Sánchez-Carrillo M, Flores-De los Ríos J, Espinosa-Medina M, Bautista-Margulis R, Ferrer-Sánchez M, Carbajal-de la Torre G, Bejar-Gómez L, Chacón-Nava J, Martinez-Villafane A (2015) Corrosion study of Fe–Al intermetallic alloys in simulated acid rain. Int J Electrochem Sci 10:2141–2154
Brito P, Schuller É, Silva J, Campos TR, de Araújo CR, Carneiro JR (2017) Electrochemical corrosion behaviour of (100), (110) and (111) Fe3Al single crystals in sulphuric acid. Corros Sci 126:366–373
Masahashi N, Kimura G, Oku M, Komatsu K, Watanabe S, Hanada S (2006) Corrosion behavior of iron–aluminum alloys and its composite steel in sulfuric acid. Corros Sci 48:829–839
Chiang W-C, Luu W-C, Wu J-K (2006) Effect of aluminum content on the passivation behavior of Fe–Al alloys in sulfuric acid solution. J Mater Sci 41:3041–3044
Banovic SW, DuPont JN, Marder AR (2000) The effect of aluminum content on the corrosion behavior of Fe–Al alloys in reducing environments at 700° C. Metall Mater Trans A 31:1805–1817
Tomaszewicz P, Wallwork GR (1983) Observations of nodule growth during the oxidation of pure binary iron–aluminum alloys. Oxid Met 19:165–185
Sukiman NL, Zhou X, Birbilis N, Hughes AE, Mol JMC, Garcia SJ (2012) Thompson GE Durability and corrosion of aluminium and its alloys: overview, property space, techniques and developments. In: Ahmad Z (ed) Aluminium alloys—new trends in fabrication and applications. InTech, Rijeka, pp 47–97
Ghali E (2010) Corrosion resistance of aluminum and magnesium alloys: understanding, performance, and testing. Wiley, New Jersey
McCafferty E (2010) Thermodynamics of corrosion: pourbaix diagrams. Introduction to corrosion science, 1st edn. Springer, New York, pp 95–117
Macdonald DD, Real S, Smedley SI, Urquidi-Macdonald M (1988) Evaluation of alloy anodes for aluminum-air batteries IV. Electrochemical impedance analysis of pure aluminum in at 25° C. J Electrochem Soc 135:2410–2414
Badawy WA, Al-Kharafi FM, El-Azab AS (1999) Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al–Cu in neutral aqueous solutions. Corros Sci 41:709–727
Macdonald JR (1987) Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. J Electroanal Chem Interfacial Electrochem 223:25–50
Hladky K, Callow LM, Dawson JL (1980) Corrosion rates from impedance measurements: an introduction. Br Corros J 15:20–25
Brug G, Van Den Eeden A, Sluyters-Rehbach M, Sluyters J (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem Interfacial Electrochem 176:275–295
Abady GM, Hilal NH, El-Rabiee M, Badawy WA (2010) Effect of Al content on the corrosion behavior of Mg–Al alloys in aqueous solutions of different pH. Electrochim Acta 55:6651–6658
Arzola-Peralta S, Llongueras JG, Palomar-Pardavé M, Romero-Romo M (2003) Study of the electrochemical behaviour of a carbon steel electrode in sodium sulfate aqueous solutions using electrochemical impedance spectroscopy. J Solid State Electrochem 7:283–288
Bhadeshia H, Honeycombe R (2017) Steels: microstructure and properties. Cambridge University Press, Cambridge
Saheb N, Laoui T, Daud AR, Harun M, Radiman S, Yahaya R (2001) Influence of Ti addition on wear properties of Al–Si eutectic alloys. Wear 249:656–662
Weng S, Huang Y, Xuan F, Luo L (2015) Correlation between microstructure, hardness and corrosion of welded joints of disc rotors. Procedia Eng 130:1761–1769
Jaradeh M, Tr Carlberg (2005) Effect of titanium additions on the microstructure of DC-cast aluminium alloys. Mater Sci Eng A 413:277–282
Acknowledgements
This work was supported by a Grant of Scientists for Next Generation (SNG) from the Academy of Scientific Research and Technology funded by Ministry of Scientific Research in Egypt, given to AHA. We acknowledge Nature Research Editing Service for language editing of the manuscript. We are thankful for the technical help received from Mohamed H. Ali.
Author information
Authors and Affiliations
Contributions
MMR, GMA, and AHA conceived of the idea. MMR supervised the work, devised the main conceptual proof outline, and was in charge of overall direction and planning. GMA performed alloys casting and helped in almost all of the technical details. AHA prepared alloys, performed all the experiments and the measurements, analysed the results, designed the figures, and wrote the manuscript. MMR and GMA verified the final draft for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Rabiei, M.M., El‑Hafez, G.M.A. & Ali, A.H. Effects of Alloying Elements (Ti and xAl) on the Electrochemical Corrosion Behaviour of Iron-Based Alloys in Corrosive Solutions of Different pH. J Bio Tribo Corros 6, 27 (2020). https://doi.org/10.1007/s40735-020-0325-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-0325-6