Abstract
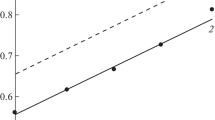
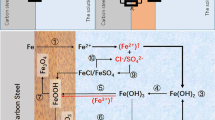
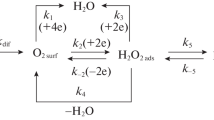
Corrosion of low-carbon steel corrosion in 2.0 M H2SO4 and 2.0 M H3PO4 containing Fe(III) salts is studied using the mass loss of metal specimens and voltammetric measurements on a rotating disk electrode. It is established that during steel corrosion in mineral acid solutions containing Fe(III) salts, the anodic ionization of metallic iron proceeds in the kinetic region. The cathodic reaction combines the parallel and independent processes of the kinetically controlled hydrogen evolution and the diffusion-controlled reduction of Fe(III) cations to Fe(II). The potentiometry and cyclic voltammetry on a platinum electrode in acid solutions containing Fe(III) salts shows that Fe(III) cations in these media are bound into complexes with acid anions. The stronger the complexes formed by Fe(III) cations, the lower their oxidation potential and diffusion coefficient. The drop in the diffusion coefficient of Fe(III) cations in mineral acid solutions affects the rate of their diffusion-controlled reduction on steel. Introducing FePO4 into a H3PO4 solution accelerates corrosion of low-carbon steel less than an equimolar Fe2(SO4)3 additive in a H2SO4 solution. The effect is a result of the lower diffusion coefficient of Fe(III) cations in a H3PO4 solution, relative to a H2SO4 solution.






Similar content being viewed by others
REFERENCES
H. Kaesche, Die Korrosion der Metalle. Physikalisch-Chemische Prinzipien und aktuelle Probleme (Springer, Berlin, 1979).
L. I. Antropov, Theoretical Electrochemistry (Vyssh. Shkola, Moscow, 1965) [in Russian].
J. O. Bockris, D. Drazic, and A. R. Despic, Electrochim. Acta 4, 325 (1961). https://doi.org/10.1016/0013-4686(61)80026-1
G. M. Florianovich, L. A. Sokolova, and Ya. M. Kolotyrkin, Electrochim. Acta 12, 879 (1967). https://doi.org/10.1016/0013-4686(67)80124-5
R. J. Chin and K. Nobe, J. Electrochem. Soc. 119, 1457 (1972).https://doi.org/10.1149/1.2404023
Ya. G. Avdeev, Int. J. Corros. Scale Inhib. 8, 760 (2019).https://doi.org/10.17675/2305-6894-2019-8-4-1
Ya. G. Avdeev and N. I. Podobaev, Korroz.: Mater., Zashch., No. 12, 25 (2004).
Ya. G. Avdeev, P. A. Belinskii, Yu. I. Kuznetsov, and O. O. Zel’, Korroz.: Mater., Zashch., No. 1, 20 (2009).
Ya. G. Avdeev, A. V. Panova, T. E. Andreeva, and Yu. I. Kuznetsov, Korroz.: Mater., Zashch., No. 11, 32 (2019). https://doi.org/10.31044/1813-7016-2019-0-11-32-40
Ya. G. Avdeev, T. E. Andreeva, and Yu. I. Kuznetsov, Int. J. Corros. Scale Inhib. 7, 366 (2018). https://doi.org/10.17675/2305-6894-2018-7-3-7
Ya. G. Avdeev, T. E. Andreeva, A. V. Panova, and E. N. Yurasova, Int. J. Corros. Scale Inhib. 8, 411 (2019). https://doi.org/10.17675/2305-6894-2019-8-2-18
S. M. Reshetnikov, Inhibitors of Acidic Corrosion of Metals (Khimiya, Leningrad, 1986) [in Russian].
Yu. V. Pleskov and V. Yu. Filinovskii, The Rotating Disk Electrode (Consultants Bureau, New York, 1976).
Yu. Yu. Lur’e, Handbook on Analytical Chemistry (Khimiya, Moscow, 1971) [in Russian].
J. M. Casas, G. Crisóstomo, and L. Cifuentes, Hydrometallurgy 80, 254 (2005). https://doi.org/10.1016/j.hydromet.2005.07.012
G. Yue, L. Zhao, O. G. Olvera, and E. Asselin, Hydrometallurgy 147–148, 196 (2014). https://doi.org/10.1016/j.hydromet.2014.05.008
M. M. Rakhimova, T. M. Nurmatov, N. Z. Yusupov, M. A. Ismailova, and E. Faizullaev, Russ. J. Inorg. Chem. 58, 719 (2013). https://doi.org/10.1134/S003602361306020X
M. M. Rakhimova, N. Z. Yusupov, K. Dzh. Suyarov, K. G. Khasanova, and Sh. Bekbudova, Russ. J. Inorg. Chem. 58, 972 (2013). https://doi.org/10.1134/S0036023613080196
V. A. Zakharov, O. A. Songina, and G. B. Bekturova, Zh. Anal. Khim. 31, 2212 (1976).
Techniques of Electrochemistry: Electrode Processes, Ed. by E. Yeager and A. J. Salkind (Wiley, New York, 1972), Vol. 1.
J. A. Plambeck, Electroanalytical Chemistry: Basic Principles and Applications (Wiley, New York, 1982).
Short Reference Book on Physicochemical Values, Ed. by K. P. Mishchenko and A. A. Ravdel (Khimiya, Leningrad, 1967), p. 103 [in Russian].
N. L. Filatova, A. G. Vendilo, and R. A. Sandu, Russ. J. Inorg. Chem. 57, 1272 (2012). https://doi.org/10.1134/S0036023612090057
Funding
This work was performed as part of the 2013–2020 Program of Basic Research for State Academies of Sciences, registration no. AAAA-A18-118121090043-0, “Developing Scientific Foundations for the Protecting Effect of Metal Corrosion Inhibitors in Gaseous and Condensed Media, Nanocomposites, and Paints and Conversion Coatings.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Avdeev, Y.G., Andreeva, T.E. Characteristics of the Mechanism of Corrosion of Low-Carbon Steels in Acid Solutions Containing Fe(III) Salts. Russ. J. Phys. Chem. 95, 1128–1136 (2021). https://doi.org/10.1134/S0036024421060029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421060029