Summary
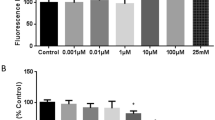
Release of arachidonic acid (AA) in brain tissue is found in various cerebral insults. Blood-brain barrier function and vasomotor response were studied during cerebral administration of the fatty acid to obtain further evidence on its role as mediator of secondary brain damage under pathological conditions. Na+-fluorescein or fluorescein isothiocyanate (FITC)-dextran were i.v. administered as low- and high-molecular weight blood-brain barrier indicators. Cortical superfusion of arachidonic acid led to moderate constriction of ca. 90% of normal of pial arteries of 60–220 μm Ø, whereas the venous diameters remained unaffected. On the other hand, AA caused opening of the blood-brain barrier not only for Na+-fluorescein but also for FITC-dextran (mol.wt. 62,000). Extravasation of Na+-fluorescein started at AA concentrations of 3×10−5 M. Concentrations of 3×10−4 to 3×10−3 M always sufficed to induce barrier opening for fluorescein, whereas 3×10−3 M was required for FITC-dextran. Leakage of the blood-brain barrier indicators started around venules. Pretreatment with indomethacin, or with BW 755 C, a dual inhibitor of both the cyclo- and lipoxygenase pathway did not prevent barrier opening by arachidonate for Na+-fluorescein. However, in the presence of indomethacin higher concentrations of AA were required to open the barrier for Na+-fluorescein, whereas BW 755 C did not influence the dose-effect relationship of AA and barrier opening observed in untreated animals. The latter findings imply that the pathophysiological effects induced by AA are likely to be attributed to the acid itself, rather than to its metabolites, a conclusion which might be in conflict with earlier observations reported in the literature. Electron microscopy revealed marked alterations of the venous endothelium, such as an attachment and eventual penetration of polymorphonuclear granulocytes through the endothelial barrier, while the small arteries and arterioles were unaffected. The findings may indicate that opening of the barrier by AA is mediated by granulocytes and/or their products. Taken together, our findings support the concept that release of AA in primarily damaged brain tissue enhances secondary processes, such as a failure of the blood-brain barrier function. The limited potency or even ineffectiveness, respectively, of indomethacin or BW 755 C provides evidence for a direct involvement of the fatty acid rather than of its metabolic degradation products. Therefore, therapeutic prevention of AA formation under these circumstances might be superior to mere inhibition of its metabolism.
Similar content being viewed by others
References
Agardh CD, Westerberg E, Siejö BK (1980) Severe hypoglycemia leads to accumulation of arachidonic acid in brain tissue. Acta Physiol Scand 190:115–116
Aritake K, Wakai S, Asano T, Takakura K, Brock M (1983) Peroxidation of arachidonic acid and brain edema. J Cereb Blood Flow Metab [Suppl 1] 3:297–298
Baethmann A (1978) Pathophysiological and pathochemical aspects of cerebral edema. Neurosurg Rev 1:85–100
Baethmann A, Kempski O, Unterberg A, Maier-Hauff K, Lange M, Schürer L (1982) Mechanismen und therapeutische Aspekte beim zerebralen Sekundärschaden. Münch Med Wochenschr 124:941–944
Bazan NG (1971) Changes in free fatty acids of brain by drug-induced convulsions, electroshock, and anesthesia. J Neurochem 18:1379–1385
Bazan NG, Rodriguez de Turco EB (1980) Membrane lipids in the pathogenesis of brain edema. Phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Adv Neurol 28:197–205
Bhakoo KK, Crockard HA, Lascelles PT (1984) Regional studies of changes in brain fatty acids following experimental ischemia and reperfusion in the gerbil. J Neurochem 43:1025–1031
Black KL, Hoff JT (1985) Leukotrienes increase blood-brain barrier permeability following intraparenchymal injections in rats. Ann Neurol 18:349–351
Brandt L, Ljunggren B, Andersson KE, Hindfelt B, Uski T (1981) Effects of indomethacin and prostacyclin on isolated human pial arteries contracted by CSF from patients with aneurysmal SAH. J Neurosurg 55:877–883
Busija DW, Heistad DD (1983) Effects of indomethacin on cerebral blood flow during hypercapnia in cats. Am J Physiol 244:519–524
Chan PH, Fishman RA (1978) Brain edema: induction in cortical slices by polyunsaturated fatty acids. Science 201:358–360
Chan PH, Fishman RA (1984) The role of arachidonic acid in vasogenic brain edema. Fed Proc 43:210–213
Chan PH, Fishman RA, Caronna J, Schmidley JW, Prioleau G, Lee J (1983a) Induction of brain edema following intracerebral injection of arachidonic acid. Ann Neurol 13:625–632
Chan PH, Kerlan R, Fishman RA (1983b) Reduction of γ-aminobutyric acid and glutamate uptake and Na+/K+-ATPase activity in brain slices and synaptosome by arachidonic acid. J Neurochem 40:309–316
Chapleau CE, White RP, Robertson JT (1980) Cerebral vasospasm: effects of prostaglandin synthetase inhibitors in vitro. Neurosurgery 6:155–159
Del Maestro RF, Thaw HH, Björk J, Planker M, Arfors KE (1980) Free radicals as mediators of tissue injury. Acta Physiol Scand [Suppl] 492:43–57
Gardiner M, Nilsson B, Rehncrona S, Siesjö BK (1981) Free fatty acids in the brain in moderate and severe hypoxia. J Neurochem 36:1500–1505
Hardebo JE, Hanko J, Owman C (1981) Possible functional importance of prostaglandin formation in cerebral arteries in response to amine receptor stimulation. J Cereb Blood Flow Metab [Suppl 1] 1:283–284
Higgs GA, Vane JR (1983) Inhibition of cyclo-oxygenase and lipoxygenase. Br Med Bull 39:265–270
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Katz AM, Messineo FC (1981) Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res 48:1–16
Koide T, Noda Y, Hata S, Sugioka K, Kobayashi S, Nakano M (1981) Contraction of the canine basilar artery following linoleic, arachidonic, 13-hydroperoxylinoleic or 15-hydroperoxyarachidonic acid. Proc Soc Exp Biol Med 168:399–402
Kontos HA (1985) Oxygen radicals in cerebral vascular injury. Circ Res 57:508–576
Kontos HA, Wei EP, Povlishock JT, Dietrich WD, Magiera CJ, Ellis EF (1980) Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science 209:1242–1245
Kuwashima J, Fujitani B, Nakamura K, Kadokawa T, Yoshida K, Shimizu M (1976) Biochemical changes in unilateral brain injury in the rat: a possible role of free fatty acid accumulation. Brain Res 110:547–557
Lundberg C, Björk J, Lundberg K, Arfors KE (1983): Polymorphonuclear leukocyte (PMNL) dependent and independent microcirculatory plasma leakage. Prog Appl Microcirc 1:86–99
Maier-Hauff K, Lange M, Schürer L, Guggenbichler C, Vogt W, Jacob K, Baethmann A (1984) Glutamate and free fatty acid concentrations in extracellular vasogenic edema fluid. In: Go KG, Baethmann A (eds) Recent progress in the study and therapy of brain edema. Plenum Press, New York, pp 183–192
Mizuno K, Yamamoto S, Land WEM (1982) Effects of nonsteroidal anti-inflammatory drugs on fatty acid cyclo-oxygenase and prostaglandin hydroperoxidase activities. Prostaglandins 23:743–757
Rehncrona S, Westerberg E, Akesson B, Siesjö BK (1982) Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem 38:84–93
Rosenblum WI (1981) Can the lipoxygenase pathway regulate cerebrovascular diameter?. J Cereb Blood Flow Metab [Suppl 1] 1:281–282
Rosenblum WI (1982) Unsaturated fatty acids and cyclooxygenase inhibitors: effects on pial arterioles. Am J Physiol 242:629–632
Sato K, Wamaguchi M, Mullan S, Evans JP, Ishii S (1969) Brain edema: a study of biochemical and structural alterations. Arch Neurol 21:413–424
Siesjö BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1:155–185
Siesjö BK, Ingvar M, Westerberg E (1982) The influence of bicuculline-induced seizures on free fatty acid concentrations in cerebral cortex, hippocampus, and cerebellum. J Neurochem 39:796–802
Smedegard G (1985) Mediators of vascular permeability in inflammation. Prog Appl Microcirc 7:96–112
Unterberg A, Wahl M, Baethmann A (1984) Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab 4:574–585
Vane JR, Ferreira SH (1979) Antiinflammatory drugs. Handb Exp Pharmacol 50, Part 2. Springer, Berlin, Heidelberg, New York
Wahl M, Unterberg A, Baethmann A (1985) Intravital fluorescence microscopy for the study of blood-brain barrier function. Int J Microcirc Clin Exp 4:3–18
Wahl M, Unterberg A, Baethmann A (1986) The effect of free radical and leukotrienes on blood-brain barrier function. Int J Microcirc Clin Exp 5:93
Wakai S, Aritake K, Asano T, Takakura K (1982) Selective destruction of the outer leaflet of the capillary endothelial membrane after intracerebral injection of arachidonic acid in the rat. Acta Neuropathol (Berl) 58:303–306
Wei EP, Ellis EF, Kontos HA (1980) Role of prostaglandins in pial arteriolar response to CO2 and hypoxia. Am J Physiol 238:226–230
Wei EP, Ellison MD, Kontos HA, Povlishock JT (1986) O2-radicals in arachidonate-induced increased blood-brain barrier permeability to proteins. Am J Physiol 251:693–699
Wolfe LS (1982) Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J Neurochem 38:1–14
Yoshida S, Abe K, Busto R, Watson BD, Kogure K, Ginsberg MD (1982) Influence of transient ischemia on lipid-soluble antioxidants, free fatty acids and energy metabolites in rat brain. Brain Res 245:307–316
Yoshida S, Inoh S, Asano T, Sano K, Shimasaki H, Ueta N (1983) Brain free fatty acids, edema and mortality in gerbils subjected to transient bilateral ischemia and effect of barbiturate anaesthesia. J Neurochem 40:1278–1286
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft Ba 452/6-5 and Wa 441/2-3
Rights and permissions
About this article
Cite this article
Unterberg, A., Wahl, M., Hammersen, F. et al. Permeability and vasomotor response of cerebral vessels during exposure to arachidonic acid. Acta Neuropathol 73, 209–219 (1987). https://doi.org/10.1007/BF00686613
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686613