Abstract
Hydrogen peroxide (H2O2) plays an important role physiologically as the second messenger and pathologically as an inducer of oxidative stress in injury, ischemia and other conditions. However, it is unclear how H2O2 influences various cellular functions in health and disease differentially, particularly in the blood-brain barrier (BBB). We hypothesized that the change in cellular concentrations of H2O2 is a major contributor in regulation of angiogenesis, barrier integrity/permeability and cell death/apoptosis in BBB endothelial cells. Rat brain microvascular endothelial cells were exposed to various concentrations of H2O2 (1 nM to 25 mM). BBB tight junction protein (zonula ocludens-1; ZO-1) localization and expression, cytoskeletal organization, monolayer permeability, angiogenesis, cell viability and apoptosis were evaluated. H2O2 at low concentrations (0.001 μM to 1 μM) increased endothelial cell tube formation indicating enhanced angiogenesis. H2O2 at 100 μM and above induced monolayer hyperpermeability significantly (p < 0.05). H2O2 at 10 mM and above decreased cell viability and induced apoptosis (p < 0.05). There was a decrease of ZO-1 tight junction localization with 100 μm H2O2, but had no effect on protein expression. Cytoskeletal disorganizations were observed starting at 1 μm. In conclusion H2O2 influences angiogenesis, permeability, and cell death/apoptosis in a tri-phasic and concentration-dependent manner in microvascular endothelial cells of the blood-brain barrier.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS) is a critical regulator of multiple body functions in human health and disease. Traumatic or ischemic injury to the brain leads to the formation of excessive (ROS) and results in oxidative stress1,2,3. This can lead to further damage to the blood-brain barrier (BBB), the primary protective barrier of the brain leading to microvascular hyperpermeability and vasogenic edema followed by several adverse consequences2,3,4. Hydrogen peroxide (H2O2) is an important endogenous ROS implicated in health and disease but its role in the BBB is not clearly known. As in the case of ROS in general, H2O2 can be beneficial or detrimental to the body but the cellular mechanisms that lead to this beneficial or adverse effects are not clearly known and are highly controversial. H2O2 has been implicated in a variety of essential cellular functions such as cell/tissue regeneration, growth, proliferation, and cell migration, while its adverse effects include damage to proteins, lipids and nucleic acid and leading to cell death5,6,7. Considering this differential and controversial nature of the effects of H2O2, and the limited information available in its effect on the BBB, we have conducted a systematic study to analyze the various effects of H2O2 in the BBB endothelial cells, the primary components of the blood brain barrier. The significance of the BBB in regulating a wide variety of human diseases including traumatic and ischemic injuries and the roles played by oxidative stress and associated signaling pathways in the pathophysiology has been the subject of active research in the recent time3,8,9. The BBB consists of interendothelial junctions and specialized transporter systems that protect the brain and maintain homeostasis. These functions are achieved through three different junctions (adherens, tight and possible gap junctions)10,11,12. Tight junctions consist of multiple types of proteins such as occludin, claudins, intracellular zonula occludens-1 (ZO-1) and junction adhesion molecules. These proteins are important in maintaining the integrity of the barrier10,13,14,15,16. Recent studies from our laboratory has shown tight junction proteins particularly ZO-1 playing a major role in maintaining barrier integrity and permeability following traumatic brain injury and tight junction disruption is critical to BBB breakdown and hyperpermeability8,13,17 Furthermore, studies from our lab as well as by others have shown oxidative stress by ROS is critical to endothelial cell barrier dysfunctions8,17,18.
Reactive oxygen species have physiologic function and are known to be important in the regulation of angiogenesis, vessel growth from preexisting vessels. Previous studies show that H2O2 induces angiogenesis19,20,21,22,23. This occurs normally during embryonic development and wound healing after surgery and trauma24, but also abnormally during carcinogenesis and metastasis. Angiogenesis is a multistep process: beginning with an increase in permeability; proliferation by growth factors; new capillary sprout elongation21,23,25; proteolysis of the basement membrane; capillary channel formation; and finally, tube stabilization. ROS is a key mediator of microvascular hyperpermeability and endothelial barrier dysfunctions in BBB8,26,27,28. It has been shown that H2O2 increases endothelial permeability through protein kinase c signaling pathway and caspase-3 activation8,29,30,31. Uncontrolled ROS formation trigged by secondary injury induces endless pool of ROS leading to massive neuronal death. Apoptosis is important for regulating the normal development and removal of damaged cells and maintain a stable environment with in the cells. H2O2 induced apoptosis may be initiated by stimulating Ca2+ dependent endonuclease activity7. H2O2 can induce apoptosis in a concentration dependent manner in cerebral vascular smooth muscle cells32 and shown to induce apoptosis in vascular endothelial cells at concentrations greater than 125 µM15,33.
Although, reactive oxygen species are considered important in the physiologic regulation and pathophysiologic dysregulation of angiogenesis, hyperpermeability, and apoptosis, their relationship or role in the BBB has not been well-studied. It is important to understand how H2O2 affects the BBB differentially to help therapeutic drug development in trauma, ischemia and in a variety of diseases, as antioxidants and ROS scavengers are widely tested as drug targets. Therefore, the purpose of this study was to test the effect of varying concentrations of H2O2 on angiogenesis, barrier functions/permeability and cell viability/apoptosis to evaluate how it affects them differentially in BBB endothelial cells.
Results
Hydrogen Peroxide Induces Concentration-dependent Change in BBB Endothelial Cells
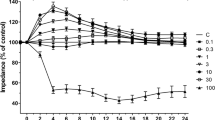
Hydrogen peroxide at 100 µM and 25 mM (2 hours) induced monolayer permeability significantly indicated by increased fluorescence intensity in media collected from the lower reservoir of the Transwell plate (p < 0.05; Fig. 1A). H2O2 at concentrations 0.001 µM to 10 µM had no significant effect on permeability compared to the untreated control group. This shows that concentrations 100 µM and above are critical in promoting oxidative stress induced loss of BBB endothelial integrity resulting in hypermeability. This study was further confirmed by trans-endothelial electrical resistance (TEER) measurement. The results from TEER measurement indicated that the lower concentrations of H2O2 (0.001 to 1 µM) showed no significant change in TEER compared to the untreated control group, whereas a significantly low resistance was observed (p < 0.05; n = 4; Fig. 1B) in cells treated with concentrations of H2O2 (10 µM) and above compared to control.
Monolayer Permeability assay demonstrating an increase in FITC-dextran fluorescence intensity as the concentration of H2O2 increases indicating barrier dysfunctions and hyperpermeability (A). Monolayer permeability is expressed as a percentage of the change in FITC-dextran-10 fluorescence intensity. ‘*’ indicates statistical significance (p < 0.05; n = 5); ‘*’ indicates significant change compared to control group (untreated group). Trans-endothelial electrical resistance measured following exposure to H2O2 showing a significant decrease in resistance as the concentration increases; ‘*’ indicates significant decrease in resistance in the cells treated with higher concentrations of H2O2 (10 µM to 25 mM) compared to control (p < 0.05; n = 6) (B).
Hydrogen Peroxide Disrupts BBB Endothelial Cell Tight Junctions
The immunofluorescence localization of tight junction protein ZO-1 demonstrated the disruption of tight junctions compared to control, whereas cells treated with 100 µM H2O2, for 2 hour, clearly indicated, disruption of cell-cell junctions and barrier damage. The disruption was observed, starting at 10 µM and was further obvious at 100 µM. Further quantitative analysis showed a significant decrease in ZO-1 localization at 100 µM concentration (p < 0.05; Fig. 2A,C) compared to all other groups (Control, 1 µM, 10 µM).
Immunofluorescence localization of tight junction protein ZO-1 and f-actin staining demonstrating disruption of the tight junctions/cytoskeletal disorganization following treatment with increasing concentration of H2O2 (for 2 hrs) in RBMECs. Treatment with 10 µM H2O2 led to clear breaks in the cell periphery/cell-cell junctions staining pattern (A, arrows) and a decrease in its localization compared to control group (C, asterisk). ‘*’ indicates statistical significance (p < 0.05; n = 4). The breakdown in the peripheral staining pattern/loss at the cell-cell junctions was more pronounced at the 100 µM treatment (A). Arrows indicate tight junction disruption. (B) Rhodamine phalloidin staining for f-actin demonstrating changes in cytoskeletal assembly evidenced by increased stress fiber formation following treatment with increasing concentration of H2O2 (for 2 hrs) in RBMECs. 1 µM and 10 µM H2O2 concentrations increased the formation of f-actin stress fibers. 100 µM showed disruption of the actin cytoskeletan (D). ‘*’ indicates statistical significance (p < 0.05; n = 4); control group. ‘**’ indicates significant change (p < 0.05) compared to control group. ‘***’ indicates significant change (p < 0.001) compared to control group (B). Arrows indicate actin stress fiber formation. Each experimental group consisted of 2 replicates. ‘Control group’ indicates an untreated group.
Hydrogen Peroxide Disrupts BBB Endothelial Cell Cytoskeletal Organization
Rhodamine phalloidin labelling showed changes in cytoskeletal assembly in response to various treatments compared to normal cells. Cells treated with low concentrations of H2O2 (starting at 1 µM and 10 µM for 2 hour), increased the formation of f-actin stress fibers compared to the control (Fig. 2B,D; p < 0.05). Treatment with H2O2 (100 µM; 2 hour) showed disruption of actin cytoskeletan and on quantitative evaluation, a significant decrease in fluorescence intensity compared to the control (Fig. 2B,D; p < 0.05).
Increasing H2O2 Concentrations Decrease Viability of BBB Endothelial cells
Treatment of H2O2 at concentrations of 10 mM and 25 mM for 2 hours resulted in significant decrease in cell viability (p < 0.05; Fig. 3) whereas concentrations of 0.001 µM, 0.01 µM, 1 µM, 10 µM 100 µM showed no significant effect on cell viability (Fig. 3).
Hydrogen peroxide treatment decreases cell viability at higher concentrations. Cell viability assay was performed to measure the number of viable (living) cells remaining after H2O2 treatment (2 hours). The cells treated with higher concentrations of H2O2 showed a significant decrease in cell viability. ‘*’ indicates statistically significant difference compared to control (p < 0.05; n = 5).
Increasing H2O2 Concentrations Cause Apoptosis of BBB Endothelial cells
Apoptosis was evaluated using two different assays. RBMECs exposed to H2O2 at 10 mM and 25 mM resulted in apoptosis compared to untreated control and other lower concentrations tested (p < 0.05; Fig. 4A). The cells exposed to the lower concentration H2O2 (0.001 to 100 µM) showed no evidence of apoptosis when evaluated using an apoptosis assay (Fig. 4A). Images were taken and fluorescence intensity was measured using image-J. The apoptosis was further confirmed using TUNEL Alexa Fluor Imaging Assay. The analysis of the data, showed that cells treated with the higher concentrations of H2O2 (10 mM and 25 mM) induced apoptosis (p < 0.05; Fig. 4B) whereas low concentrations (10 µM and 100 µM) showed no evidence of apoptosis.
Hydrogen peroxide treatment increases apoptosis at higher concentrations evaluated by caspase-3/7 apoptosis assay (A). H2O2 at 10 mM and 25 mM induces apoptosis ‘*’ indicates statistically significant increase compared to control group (p < 0.05; n = 4). Hydrogen peroxide treatment increases apoptosis at higher concentrations when studied using TUNEL assay (B; n = 5). H2O2 at 10 mM and 25 mM induces apoptosis (some of the apoptotic cells are indicated by white arrows).
Hydrogen Peroxide Induces Angiogenesis in Blood-Brain Barrier Endothelial cells
The change in angiogenesis was visualized and further quantitatively measured using image-J. Treatment with the lower concentrations of H2O2 (0.001 to 1 µM) resulted in significant increase in total endothelial cell tube length compared to the control (p < 0.05; Fig. 5A,B).
Effect of increasing concentrations of H2O2 on angiogenesis. The cells were treated with increasing concentrations of H2O2 (2 hrs) after seeding them in Matrigel and observed after 4 hrs. The cells treated with lower concentrations (0.001 µM, 0.01 µM, 1 µM) of H2O2 showed a significantly increase in angiogenesis (A). ‘*’ indicates statistically significant difference compared to control/untreated group (p < 0.05; n = 3). The lower panel (B) shows original images.
Effects of H2O2 on Tight Junction Protein Expression
Immunoblot analysis showed the presence of tight junction protein ZO-1 (Fig. 6). Hydrogen peroxide treatment from 0.01 to 10 mM showed no significant change in ZO-1 protein expression. ZO-1 protein was normalized to the expression of β-actin for quantitative analysis (n = 5).
Immunoblot analysis of the tight junction protein ZO-1 demonstrating the effect of increasing concentrations of H2O2 on ZO-1 protein expression in RBMECs (A and B). Hydrogen peroxide treatment had no effect on ZO-1protein expression at any of the tested concentrations. The protein was normalized to the expression of β-actin (n = 5). The original images are given as supplementary figures (Supplementary Figs 3 and 4).
Evaluation of the effects of H2O2 on endothelial cell senescence
H2O2 is known to induce pre-mature senescence in cells. This study was conducted to determine if the hyperpermeability observed (with 100 µM and above) was due to H2O2-induced pre-mature senescence in RBMECs. Senescent cells normally display increase of cell size and senescence-associated expression of β-galactosidase (SA-β-Gal) activity. The results show that H2O2 treatment didn’t induced pre-mature senescence compared to the control as there was no SA-β-Gal activity observed. The cells exposed to H2O2 (100 µM) for 2 hours and maintained for 72 hours showed evidence of senescence/SA-β-Gal activity (Supplementary Fig. 1).
Visualization of intracellular H2O2
Intracellular H2O2 levels were visualized to confirm the presence of H2O2 during the treatment period in RBMECs. The results show that H2O2 is present intracellularly following treatment of H2O2 (Supplementary Fig. 2).
Discussion
The purpose of the study was to evaluate the relationship between angiogenesis, permeability and cell death/apoptosis in BBB endothelial cells following H2O2 exposure to understand why H2O2 behave differentially in these conditions. The important observation of the study are: (1) low levels of H2O2 can cause angiogenesis (2) H2O2 plays an important role in promoting microvascular permeability by inducing tight junction disruption and cytoskeletal disorganization as its levels increases; (3) H2O2 when reaches a higher cellular level promotes cell death/apoptosis; (4) H2O2-mediated cellular changes are dependent on the tight junction organization evidenced by changes in Zonula Occludens-1 (ZO-1) and cytoskeletal assembly. All these effects are dependent on the changes in cellular levels of H2O2. Altogether these results demonstrate a tri-phasic role for H2O2 in regulating multiple cellular functions in brain microvascular endothelial cells, the primary components of the BBB and explain the basis of the various cellular manifestations subjected to changing levels of cellular H2O2 and possibly the oxidative stress status of these cells.
Although H2O2 has been previously shown to induce a wide variety of cellular functions such as angiogenesis, permeability and cell death, their relationship or underlying cellular mechanisms are not clearly known. Furthermore, the role played by H2O2 in regulating BBB-related functions remained unknown. The implications of our findings imply to a wide variety of disease conditions where oxidative stress and/or H2O2 play a significant role in its pathophysiology such as in traumatic and ischemic brain injury, multiple sclerosis, Alzheimer’s disease and Parkinson’s disease24,34,35. In addition to this, impairment of the BBB has been implicated in all the above conditions8,13,17.
Angiogenesis is an important function for growth, wound healing, and in the pathologic process of tumorigenesis19,36. Several studies have shown that H2O2 stimulates endothelial cell proliferation in different cell types but brain microvascular endothelial cells, the primary components of the BBB remained an unexplored area. Our study utilized isolated rat brain microvascular endothelial cells cultured on Matrigel, comparable to normal angiogenesis process in the brain. Endothelial tube formation is considered a critical step in in vitro angiogenesis. After the exposure to H2O2, a significant increase in tube formation was observed compared to the control group, demonstrated that low concentration of H2O2 can cause angiogenesis whereas the higher concentrations of H2O2 inhibit the tube formation.
Blood-brain barrier breakdown leads to microvascular hyperpermeability, the excessive leakage of proteins and fluid from the intravascular space to the insterstitium10,13,17,37. Clinically this is manifested as cerebral edema, increased intracranial pressure, and brain herniation38,39,40. Our results explain how H2O2 promotes microvascular permeability in the blood-brain barrier. Hydrogen peroxide at a concentration of 100 µM lead to increased permeability, decreased trans-endothelial electrical resistance (started observing from 10 µM) and also showed disruption of the tight junctions evidenced by ZO-1 localization, tight junction morphology and organization. However, none of the concentrations included in this study led to a change in overall ZO-1 protein levels. This could be due to the reason that higher H2O2 concentrations resulted in promoting the dislocation of ZO-1 from the tight junctions without affecting the protein expression/content. This was further supported by our observation that f-actin stress fibers were increased parallel to ZO-1 dislocation. This is likely secondary to the change in ZO-1 localization at the tight junctions, as it serves as a scaffolding protein between tight junction proteins and actin cytoskeletal assembly.
Although there are many adverse effects for H2O2, its beneficial effect on brain endothelial cells adds to its therapeutic potential for many types of neurological pathologies10. The effect of H2O2 on cell death/apoptosis has been observed in a variety of cell types. Most of these studies looked at longer exposure times and found that H2O2 concentrations between 50 µM and 500 µM induced apoptosis and even higher concentrations led to unregulated cell death, necrosis41,42,43,44. Our study was conducted to determine the basis of its effect in a comparative manner and also in the context of the BBB testing it in rat brain microvascular endothelial cells. Apoptosis was evaluated by a caspase-3/7 assay and TUNEL assay. In this study H2O2 at a higher concentration of 10 mM, led to apoptosis and decreased cell viability. Although this suggest the adverse effects H2O2, this programmed cell death is important in wound healing and the prevention of tumorigenesis7,45. In our study, hydrogen peroxide at 100 µM induced hyperpermeability but did not decrease cell viability or increased apoptotic cell death. This suggests that permeability occurs independently of cell death. The increase in permeability could be attributed to changes in barrier integrity and compromised cytoskeletal organization. Although H2O2 at 25 mM decreased cell viability and 100 µM did not, their effect on permeability remained comparable. It is possible that permeability due to H2O2 at 100 µM reached its maximum and any further increase was not possible. This is evident from the data from the TEER study as well.
In order to determine if H2O2-induced-hyperpermeability is related to potential pre-mature senescence of endothelial cells, we conducted a senescence assay. Our results show that the concentrations of H2O2 that induce hyperpermeability doesn’t induce pre-mature senescence in endothelial cells. The ability of cells to remove extracellular H2O2 may vary between different cell types/cell lines. We conducted an H2O2 visualization assay to demonstrate intracellular presence of H2O2 following exposure to H2O2. Our results show the presence of detectable H2O2 intracellularly during the treatment period.
Our results clearly demonstrate a concentration dependent effect of H2O2 in regulating three significant cellular effects such as permeability regulation, angiogenesis and cell viability. As with any experiment, there are limitations of this study. This is an in vitro study conducted in rat brain microvascular endothelial cells and with increasing complexity with the small animal or human models, it is not known how H2O2 differentially regulates these functions. Rat brain microvascular endothelial cells are ideal tools for evaluating blood-brain barrier-related studies. The clinical implications of this study are unclear at this point. In a clinical context, considering the extensive use of non-prescription antioxidant medications and identification of newer antioxidant therapeutic drugs for various ailments, this study merits immense significance. In the meantime, H2O2 being a cellular signaling molecule, this study provides a clear basis for its multiple but differential effects in the blood-brain barrier. The tight junctions of endothelial cells are the most important barrier component of the BBB; however, this is a complex system made up of pericytes, astrocytes, and neurons and the monolayer permeability assay is an imperfect simulation. In conclusion, H2O2 has a tri-phasic role in rat brain microvascular endothelial cells and the understanding of these physiologic and pathologic effects will aid in the study of the BBB and neurovascular pathologies and in conditions such as traumatic brain injury and ischemic stroke.
Materials and Methods
Chemicals and Reagents
Rat Brain microvascular endothelial cells and Media were purchased from Cell Applications, Inc. (San Diego, CA). Transwell insert plates (24 well) were obtained from Corning, USA. Fibronectin (5%) from bovine plasma, chamber slides and FITC-Dextran-10 were purchased from Sigma Aldrich (St. Louis, MO, USA). DMEM, MEM, ZO-1 primary antibody, rhodamine phalloidin, Cell Event Caspase-3/7 Green Detection Reagent, Pierce BCA protein assay kit and he Click-iT® TUNEL Alexa Fluor® 488 Imaging Assay Kit were purchased from ThermoFisher Scientific (Waltham, MA, USA). Angiogenesis assay kit was bought from EMD Millipore/Calbiochem (Billerica, MA, USA). Cell viability assay kit, Senescence assay Kit and intracellular H2O2 detection Kit were purchased from Bio vision (Milpitas, CA, USA).
Endothelial Cell Culture
Primary cultures of rat brain microvascular endothelial cells obtained commercially, as described above, were grown on fibronectin coated cell culture dishes, using rat brain endothelial media in optimal cell culture condition (95% O2, 5% CO2 at 37 °C). Once the cells attain 60–80% confluency, cells were transferred to Transwell inserts, chamber slides, 96 well plate or regular dishes as appropriate for experimental purposes. For all the experiments, cells of 6–9 passages were utilized.
Monolayer Permeability Assay
RBMECs were grown on Transwell membranes coated with fibronectin until it formed a monolayer. It usually takes 4–5 days to form a monolayer. They were exposed to different concentrations of H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 25 mM) for 2 hours. After treatment the FITC-dextran (10 kDa) was applied to the upper chamber incubate for 30 minutes. Samples were collected from the lower chamber. The Fluorescence intensity of the samples were measured using spectrophotometer. Each experimental group consisted of five replicates
Trans Endothelial Electrical Resistance (TEER) Measurement
Transendothelial electrical resistance was measured using EVOM2. Rat brain microvascular endothelial cells were grown on Transwell membranes for 4 days and then exposed to different concentrations of H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 10 mM and 25 mM) for 2 hours as described above. After the treatment with H2O2 TEER was measured. The changes in resistance was calculated as percentage (%) control.
Immunofluorescence localization and cytoskeletal labeling
Rat brain microvascular endothelial cells were grown on 8-well chamber slides coated with fibronectin for 24 hours followed by treatment with different concentrations of H2O2 (1 µM, 10 µM, 100 µM, 2 hours). The cells were washed with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 15 minutes. After fixation the cells were permeabilized using 0.25% Triton-X 100 in PBS followed by blocking with 2% BSA in PBS for one hour at room temperature. For immunofluorescence study, the cells were incubated with primary antibody for ZO-1 for overnight, and the cells were incubated with secondary antibody for one hour in room temperature followed by washing in PBS and a mounting medium containing DAPI. For f-actin labelling, cells were exposed to rhodamine phalloidin for 20 minutes followed by washing and mounting as describe above. Cells were observed under a Confocal Microscope (60X water immersion objective).
Cell Viability Assay
Rat brain microvascular endothelial cells were grown on black 96 well plates for 24 hours. Once the cells formed a monolayer, they were washed in PBS and exposed to phenol red-free medium for 1 hour. Cells were then treated with different concentrations of H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 10 mM and 25 mM; 2 hours). After the treatments, Calcein buffer solution was applied to the cells and incubated at 37 °C for 30 minutes and a fluorescence intensity was measured. Each experimental group consisted of 5 replicates. Fluorescence intensity was expressed as percentage of the control.
Apoptosis Assay
Cell Event Caspase-3/7 Green Detection Reagent was used to study cellular apoptosis with exposure to different concentrations of H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 10 mM, 25 mM; 2 hours). This reagent is a four amino acid peptide, DEVD, conjugated to a nucleic acid binding dye with absorption/emission maxima of ~502/530 nm. The DEVD peptide sequence is a cleavage site for caspase-3/7 and the conjugate dye is non-fluorescent until cleaved from the peptide and bound to DNA. Therefore, the reagent is non-fluorescent until activation of caspase-3/7 in apoptotic cells leads to cleavage of the DEVD peptide. This allows the dye to bind to DNA and produce bright, fluorogenic nuclei. RBMECs were grown on chamber slides as described above and divided into control (untreated) and different concentrations of H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 10 mM, 25 mM; 2 hours). Following this, Cell Event Caspase-3/7 Apoptosis Detection Reagent was added and cells were incubated at 37 °C for 30 minutes. Cells were then visualized and scanned at a single optical plane with Olympus Confocal Microscope. Each experimental group consisted of 4 replicates.
TUNEL Alexa Fluor Imaging Assay
Tunnel Assay was conducted to measure apoptotic cells. RBMECs were grown on black 96-well plates with clear bottom. The cells were exposed to different concentrations of H2O2 (10 µM, 100 µM, 10 mM, 25 mM) for 2 hours. Cells were washed in PBS and then fixed with 4% para formaldehyde in PBS and permeabilized with 0.25% Triton X-100. TdT (terminal deoxynucleotidyl transferase) reaction mixture was added to the cells followed by Click-iT® Reaction Cocktails. Cells were washed in 3% BSA in PBS and observed under a Confocal Microscope.
Angiogenesis Assay
Angiogenesis assays were performed by using RBMEC grown in RBMEC culture media. The cells were harvested and the cell suspensions containing 150,000 cells in 150 µl of RBMEC media and were seeded in triplicates on Matrigel coated glass bottom 96 well plates. Then they were treated with different concentrations of H2O2 for 2 hrs. After seeding the cells, the plates were incubated for 4 hrs at 37 °C and 5% CO2. The cells were observed after 4 hr. Tube formation was visualized using a phase contrast inverted (confocal) microscope 20X magnification. Quantification of Tube Network was done using Image J with the Angiogenesis Analyzer plugin16. The results are expressed as mean ± SD.
Western blot Analysis
RBMECs were grown on 100 mm dishes. After confluency of 80%, the cells were divided into untreated (control) and treated group. The cells were treated with different concentration of H2O2 for 2 hrs at 37 °C. Standard western blot was performed to determine the expression of ZO-1 in RBMEC. The samples were incubated with the primary mouse monoclonal anti ZO-1 antibody (1:250 dilutions) overnight at 4 °C and then incubated with the goat anti-mouse IgG-HRP conjugated secondary antibody. The each experiment was repeated five times. Equal amount of protein sample loading was verified by assessing β-actin expression.
Senescence Detection Assay
Hydrogen peroxide is known to induce pre-mature senescence in cells. In order to determine, if H2O2-induced hyperpermeability was independent of pre-mature senescence of endothelial cells, cellular senescence was evaluated using a senescence detection assay kit, Senescent cells normally display increase of cell size and senescence-associated expression of β-galactosidase (SA-β-Gal) activity. The cells were grown on 12-well plates and exposed to different concentrations of H2O2 (10 µM, 100 µM and 10 mM; 2 hours) at 37 °C. The cells were washed in PBS, and fixed with a fixative solution for 15 minutes. After that the cells were washed twice in PBS, the staining solution was added to the cells and incubated overnight at 37 °C. The cells were observed under a microscope for bright field imaging for increased cell size and increased SA-β-Gal activity evident by a blue staining. As a positive control, the cells were treated with H2O2 for 2 hours, replaced with fresh media and maintained for 72 hours prior to SA-β-Gal staining. Long-duration H2O2 exposure is known to induce pre-mature senescence in endothelial cells.
Visualization of intracellular H2O2
This assay was performed to confirm the presence of H2O2 intracellularly following exposure to H2O2. Intracellular hydrogen peroxide detection kit uses a dye that reacts with intracellular hydrogen peroxide to produce an orange color and fluorescence, which is proportional to the concentration of intracellular hydrogen peroxide. RBMECs (2–3 × 104 cells/well) were grown on a 96-well plate. Cells were incubated overnight in 37 °C incubator containing 5% CO2. Next day, cells were treated with different concentration H2O2 (0.001 µM, 0.01 µM, 1 µM, 10 µM, 100 µM, 10 mM, 25 mM) or control for 2 hour in the presence of the Dye. Cells were examined using a fluorescence microscope (Excitation/Emission = 543 nm/545–750 nm). Images were acquired from each well.
Statistical Analysis
The data from all experiments were analyzed and expressed as the mean +/− SEM (%).One-way analysis of variance (ANOVA) followed by Bonferroni test was performed to identify the groups that are statistically different (P < 0.05).
References
Quillinan, N., Herson, P. S. & Traystman, R. J. Neuropathophysiology of Brain Injury. Anesthesiol Clin 34, 453–464 (2016).
Lochhead, J. J., Ronaldson, P. T. & Davis, T. P. Hypoxic Stress and Inflammatory Pain Disrupt Blood-Brain Barrier Tight Junctions: Implications for Drug Delivery to the Central Nervous System. AAPS J 19, 910–920 (2017).
Cruz-Haces, M., Tang, J., Acosta, G., Fernandez, J. & Shi, R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener 6, 20 (2017).
Nizamutdinov, D. & Shapiro, L. A. Overview of Traumatic Brain Injury: An Immunological Context. Brain Sci 7 (2017).
Maulik, N. Redox signaling of angiogenesis. Antioxid. Redox Signal. 4, 805–815 (2002).
Yun, J. et al. Redox-dependent mechanisms in coronary collateral growth: the ‘redox window’ hypothesis. Antioxid. Redox Signal. 11, 1961–1974 (2009).
Lennicke, C., Rahn, J., Lichtenfels, R., Wessjohann, L. A. & Seliger, B. Hydrogen peroxide – production, fate and role in redox signaling of tumor cells. Cell Communication and Signaling 13, 39 (2015).
Alluri, H. et al. Reactive oxygen species-caspase-3 relationship in mediating blood-brain barrier endothelial cell hyperpermeability following oxygen-glucose deprivation and reoxygenation. Microcirculation 21, 187–195 (2014).
Luissint, A.-C., Artus, C., Glacial, F., Ganeshamoorthy, K. & Couraud, P.-O. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids and Barriers of the CNS 9, 23 (2012).
Goldstein, N. et al. Blood-brain barrier unlocked. Biochemistry Mosc. 77, 419–424 (2012).
Nitta, T. et al. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J Cell Biol 161, 653–660 (2003).
Weidinger, A. & Kozlov, A. V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 5, 472–484 (2015).
Alluri, H. et al. Attenuation of Blood-Brain Barrier Breakdown and Hyperpermeability by Calpain Inhibition. J. Biol. Chem. 291, 26958–26969 (2016).
Stamatovic, S. M., Johnson, A. M., Keep, R. F. & Andjelkovic, A. V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4, e1154641 (2016).
Granger, D. N. & Kvietys, P. R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol 6, 524–551 (2015).
Freeman, L. R. & Keller, J. N. Oxidative stress and cerebral endothelial cells: Regulation of the blood–brain-barrier and antioxidant based interventions. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822, 822–829 (2012).
Alluri, H. et al. Melatonin Preserves Blood-Brain Barrier Integrity and Permeability via Matrix Metalloproteinase-9 Inhibition. PLOS ONE 11, e0154427 (2016).
Tharakan, B., Whaley, J. G., Hunter, F. A., Smythe, W. R. & Childs, E. W. -)-Deprenyl inhibits vascular hyperpermeability after hemorrhagic shock. Shock 33, 56–63 (2010).
Huang, S.-S. & Zheng, R.-L. Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie 61, 223–229 (2006).
Mu, P., Liu, Q. & Zheng, R. Biphasic regulation of H2O2 on angiogenesis implicated NADPH oxidase. Cell Biol. Int. 34, 1013–1020 (2010).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Yasuda, M. et al. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 64, 249–258 (1999).
Yoo, S. Y. & Kwon, S. M. Angiogenesis and Its Therapeutic Opportunities. Mediators Inflamm 2013 (2013).
McCord, J. M. Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 26, 351–357 (1993).
Apoptosis, Cell Signaling, and Human Diseases: Molecular Mechanisms, Volume 2. (Humana Press, 2010).
Lewén, A., Matz, P. & Chan, P. H. Free radical pathways in CNS injury. J. Neurotrauma 17, 871–890 (2000).
Pun, P. B. L., Lu, J. & Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 43, 348–364 (2009).
Chen, X. et al. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience 226, 89–100 (2012).
Kevil, C. G. et al. Role of cadherin internalization in hydrogen peroxide-mediated endothelial permeability. Free Radic. Biol. Med. 24, 1015–1022 (1998).
Siflinger-Birnboim, A., Goligorsky, M. S., Del Vecchio, P. J. & Malik, A. B. Activation of protein kinase C pathway contributes to hydrogen peroxide-induced increase in endothelial permeability. Lab. Invest. 67, 24–30 (1992).
Siflinger-Birnboim, A. & Malik, A. B. Regulation of endothelial permeability by second messengers. New Horiz 4, 87–98 (1996).
Li, J. et al. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: possible relation to neurodegenerative diseases and strokes. Brain Res. Bull. 62, 101–106 (2003).
Kim, Y.-W. & Byzova, T. V. Oxidative stress in angiogenesis and vascular disease. Blood 123, 625–631 (2014).
Cohen, G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann. N. Y. Acad. Sci. 738, 8–14 (1994).
Halliwell, B. & Gutteridge, J. M. Free radicals and antioxidant protection: mechanisms and significance in toxicology and disease. Hum Toxicol 7, 7–13 (1988).
Lee, H. S., Han, J., Bai, H.-J. & Kim, K.-W. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 276, 4622–4635 (2009).
Werner, C. & Engelhard, K. Pathophysiology of traumatic brain injury. Br J Anaesth 99, 4–9 (2007).
Bains, M. & Hall, E. D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822, 675–684 (2012).
Larochelle, C., Alvarez, J. I. & Prat, A. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Letters 585, 3770–3780 (2011).
Zahedi, K. et al. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 27, 515–525 (2010).
Teramoto, S. et al. Hydrogen Peroxide-Induced Apoptosis and Necrosis in Human Lung Fibroblasts: Protective Roles of Glutathione. The Japanese Journal of Pharmacology 79, 33–40 (1999).
Saito, Y. et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic. Res. 40, 619–630 (2006).
Clément, M.-V., Ponton, A. & Pervaiz, S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Letters 440, 13–18 (1998).
Park, W. H. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. International Journal of Molecular Medicine 31, 471–476 (2013).
López-Lázaro, M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 252, 1–8 (2007).
Author information
Authors and Affiliations
Contributions
C.A.S. and B.T. conceived, designed and coordinated the study and wrote the paper. C.A.S., A.Y. and B.D.R. designed and performed the experiments and analyzed the data; C.A.S. provided technical assistance and contributed to the preparation of the figures and manuscript. C.A.S., B.D.R., M.R.B., B.T., C.L.I., J.H.H. and M.L.D. were involved in the conception, experimental design and manuscript preparation. All authors reviewed the results and approved (except M.L.D. who passed away before finalizing the manuscript), the final version of the manuscript. We acknowledge Ms. Anna Webb for help with imaging and its analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anasooya Shaji, C., Robinson, B.D., Yeager, A. et al. The Tri-phasic Role of Hydrogen Peroxide in Blood-Brain Barrier Endothelial cells. Sci Rep 9, 133 (2019). https://doi.org/10.1038/s41598-018-36769-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36769-3
- Springer Nature Limited
This article is cited by
-
Effects of acute and chronic oxidative stress on the blood–brain barrier in 2D and 3D in vitro models
Fluids and Barriers of the CNS (2022)
-
Brain microvascular endothelial cell dysfunction in an isogenic juvenile iPSC model of Huntington’s disease
Fluids and Barriers of the CNS (2022)
-
Microglia as the Critical Regulators of Neuroprotection and Functional Recovery in Cerebral Ischemia
Cellular and Molecular Neurobiology (2022)
-
Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway
Acta Pharmacologica Sinica (2021)