Summary
Hypopomus occidentalis is a weakly electric Gymnotiform fish with a pulse-type electric organ discharge (EOD).Hypopomus used in this study were taken from one of the northernmost boundaries of this species, the Atlantic drainage of Panama where the animals breed at the beginning of the dry season (December). In normal breeding populations,Hypopomus occidentalis exhibit a sexual dimorphism in EOD and morphology. Mature males are large with a broad tail and have an EOD characterized by a low peak power frequency. Females and immature males are smaller, having a slender tail and EODs with higher peak power frequencies (Fig. 1). This study describes differences in the EOD and electric organ morphology between breeding field populations of male and femaleHypopomus. Changes in physiology, morphology and EOD shape which may accompany this seasonal change were examined in steroid injected fish, using standard histological and physiological techniques.
A group of females were injected with hormones (5α-dihydrotestosterone (DHT), estrogen or saline) to assess changes in their morphology and EOD. Animals treated with DHT developed characteristics which mimicked the sexually dimorphic characteristics of a male, while the other groups did not (see Fig. 5). Tissue from the tails of breeding males and females, and females treated with DHT, were sampled to measure the size of the electrocytes in the tail. The broader tail of males and DHT-females is composed of large electrocytes, whereas the slender tail of normal females is composed of smaller electrocytes. Therefore, the increase in the tail width in the female DHT group is caused by an enlargement of the electrocytes in this area.
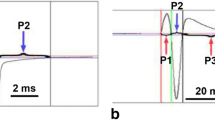
Intracellular recordings from the electrocytes of saline and DHT injected females show a difference in the responses of the rostral faces of the electrocytes from the two groups, which reflect the differences in their EODs. Saline-treated animals had symmetrical EODs (the first and second phase of the EOD were equal in duration and amplitude), while the physiological responses from each face of the electrocytes yielded responses that were similarly equal in duration and amplitude. DHT-treated animals had asymmetrical EODs (the first phase of the EOD was similar to that of saline treated fish and larger in amplitude and shorter in duration than the second phase) and the physiological responses of the electrocytes reflected this asymmetry. The differential recordings across the caudal face were similar to those from saline treated fish, while the responses from the rostral face were longer in duration and smaller in amplitude.
These data suggest that the effects of androgens underlie the changes in single electrocytes which produce the sexually dimorphic signals and morphology present in natural breeding populations ofHypopomus occidentalis.
Similar content being viewed by others
References
Bardin CW, Catterall JF (1981) Testosterone: a major determinant of extragenital sexual dimorphism. Science 211:1285–1294
Bass AH, Denizot J-P, Hopkins, CD (1983) Electric organ morphology of mormyrids: Substrates for species and sex differences in the electric organ discharge. Anat Rec 205:16A
Bass AH, Hopkins CD (1983) Hormonal control of sexual differentiation: Changes in electric organ discharge waveform. Science 220:971–973
Bass AH, Hopkins CD (1984) Shifts in frequency tuning in androgen-treated mormyrid fishes. J Comp Physiol 155:713–724
Bass AH, Hopkins CD (in press) Hormonal control of sex differences in the electric organ discharge (EOD) of mormyrid fishes. J Comp Physiol
Bass AH, Segil N, Kelley DB (1984) A steroid sensitive electromotor pathway in mormyrid fish: Electric organ morphology, androgen receptor biochemistry and steroid autoradiography. Neurosci Abstr 10:927
Bennett MVL (1961) Modes of operation of electric organs. Ann NY Acad Sci 94:458–509
Bennett MVL (1971) Electric organs. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, pp 347–491
Bennett MVL, Grundfest H (1959) Electrophysiology of electric organs inGymnotus carapo. J Gen Physiol 42:1067–1104
Bennett MVL, Grundfest H (1966) Analysis of depolarizing and hyperpolarizing inactivation responses in Gymnotid electroplaques. J Gen Physiol 50:141–169
Black-Cleworth P (1970) The role of electric discharges in the non-reproductive social behavior ofGymnotus carapo. Anim Behav Monogr 3:1–77
Boulenger GA (1907) Zoology of Egypt: the fishes of the Nile. Hugh Rees Limited, London, pp 1–578
Gottschalk B (1981) Electrocommunication in Gymnotoid wave fish: significance of a temporal feature in the electric organ discharge. In: Szabo T, Czeh G (eds) Sensory physiology of aquatic lower vertebrates. Pergamon Press, Budapest, pp 225–278
Hagedorn M, Heiligenberg W (1985) Court and spark: electric signals in the courtship and mating of gymnotoid fish. Anim Behav 33:254–265
Heiligenberg W (1977) Principles of electrolocation and jamming avoidance in electric fish. Studies in brain function I. Springer, Berlin Heidelberg New York, pp 1–87
Hopkins CD (1974a) Electric communication in the reproductive behavior ofSternopygus macrurus (Gymnotoidae). Z Tierpsychol 35:518–535
Hopkins CD (1974b) Electric communication: Functions in the social behavior ofEigenmania virescens. Behaviour 50:270–306
Hopkins CD (1974c) Electrocommunication in fish. Am Sci 62:426–437
Hopkins CD (1980) Evolution of electric communication channels of mormyrids. Behav Ecol Sociobiol 7:1–13
Hopkins CD (1981) On the diversity of electric signals in a community of mormyrid electric fish in West Africa. Am Zool 21:211–222
Hopkins CD (1983) Functions and mechanisms in electrolocation. In: Northcutt G, Davis R (eds) The central nervous system of teleost fishes. The University of Michigan Press, Ann Arbor, pp 215–259
Hopkins CD, Bass AH (1981) Temporal coding of species recognition signals in an electric fish. Science 212:85–87
Iles RB (1960) External sexual differences and their significance inMormyrus kannumme Forskal 1775. Nature 188:516
Mazeaud MM, Mazeaud F, Donaldson EM (1977) Primary and secondary effects of stress in fish: some new data with a general review. Trans Am Fish Soc 106 (3): 201–211
Meyer JH (1983) Steroid influences upon the discharge frequencies of a weakly electric fish. J Comp Physiol 153:29–37
Meyer JH, Zakon HH (1982) Androgens alter the tuning of electroreceptors. Science 217:635–637
Sokal RR, Rohlf FJ (1969) Biometry: the principles and practice of statistics in biological research. Freeman, San Francisco
Westby GWM (1974) Further analysis of the individual discharge characteristics predicting social dominance in the electric fish,Gymnotus carapo. Anim Behav 23:249–260
Westby GWM, Kirschbaum F (1977) Emergence and development of the electric organ discharge in the mormyrid fish,Pollimyrus isidori. I. The larval discharge. J Comp Physiol 122:251–271
Westby GWM, Kirschbaum F (1978) Emergence and development of the electric organ disharge in the mormyrid fish,Pollimyrus isidori. II. Replacement of the larval by the adult disharge. J Comp Physiol 127:45–59
Westby GMW, Kirschbaum F (1981) Sex differences in the electric organ discharge ofEigenmannia virescens and the effect of gonadal maturation. In: Szabo T, Czeh G (eds) Sensory physiology of aquatic lower vertebrates, vol 3. Pergamon Press, Budapest, pp 179–194
Westby GMW, Kirschbaum F (1982) Sex differences in the waveform of the pulse-type electric fish,Pollimyrus isidori (Mormyridae). J Comp Physiol 145:399–403
Zakon HH, Meyer JH (1983) Plasticity of electroreceptor tuning in the weakly electric fish,Sternopygus dariensis. J Comp Physiol 153:477–487
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hagedorn, M., Carr, C. Single electrocytes produce a sexually dimorphic signal in South American electric fish,Hypopomus occidentalis (Gymnotiformes, Hypopomidae). J. Comp. Physiol. 156, 511–523 (1985). https://doi.org/10.1007/BF00613975
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613975